Abstract
Malignancy is an established risk factor for thromboembolism. Various chemotherapeutic agents increase the risk of venous and arterial thromboembolism. Though venous thromboembolism has been reported, arterial thrombosis is not widely described in the literature. Patient with cervical cancer treated with cisplatin-based concurrent radiation-chemotherapy diagnosed with arterial thrombosis has not yet been reported. We report two cases of acute mesenteric and celiac arterial thrombosis. Often the unusual complications like arterial thrombosis go undetected or get delayed resulting in a poor prognosis. Arterial thrombosis can be life-threatening, and prompt diagnosis is important to start timely, adequate treatment. The treating physician should have a high level of clinical suspicion and a low threshold for imaging so that early diagnosis of vascular complications in carcinoma cervix patients getting cisplatin-based concurrent chemoradiotherapy can be done.
Introduction
Malignancy is an established risk factor for thromboembolism [1]. It increases the risk of thrombosis by 4.5 times, and this risk increases to 6 times in patients receiving chemotherapy as various chemotherapeutic agents multiply the risk of venous and arterial thromboembolism [2]. Increased risk of arterial thrombosis in malignancy is multifaceted, but several factors such as type of malignancy, hypertension, obesity, atherosclerosis, smoking, and hereditary conditions compound the risk [3]. Pathogenesis of arterial thrombosis is different from venous, but a subclinical hypercoagulable state combined with prothrombotic chemotherapy drugs substantially increases the risk [3].
Arterial thrombosis in cancer patients can be life-threatening and can lead to poor outcomes even with adequate treatment. Patients with newly diagnosed cancer have an increased risk of arterial thromboembolism, especially during the first six months of diagnosis [4]. Most of the literature reports Thromboembolic Events (TEE) associated with lung, hematological and gastrointestinal malignancies. Though venous thromboembolism has been commonly reported, arterial thrombosis is not widely described in the literature. Thromboembolic events have not been reported routinely in patients with cervical malignancy treated with radiation with concurrent cisplatin chemotherapy. Retrospective analysis has reported accelerated arterial and venous thrombosis in patients treated with cisplatin-based chemotherapy [5]. We hereby report two cases of acute arterial thrombosis in patients with cervical cancer during the course of cisplatin-based radiation chemotherapy.
Case Presentation 1
A 42-year-old lady having no documented comorbidities and addictions with no past and family history of any TEE/ predisposing disorders were diagnosed with cervical cancer FIGO stage IIIB. She had five full-term normal vaginal deliveries with no significant events. Clinically, the left parametrium was involved medially, and the right parametrium up to a lateral pelvic wall. Histology was moderately differentiated squamous cell carcinoma; Index contrast-enhanced Computed Tomography (CT) scan of abdomen and pelvis showed localized disease with right-sided external iliac lymph nodes measuring 1.8 cm x 1.0 cm and no para-aortic nodes. She was planned for definitive chemo-radiotherapy in view of her locally advanced disease. During the course of treatment, after completion of 16 fractions of radiotherapy (to the pelvis only) and two cycles of cisplatin chemotherapy (weekly 40 mg/m2) (Dose per cycle was 60 mg), she presented to the emergency department with sudden onset acute abdominal pain, not relieved by intravenous analgesics.
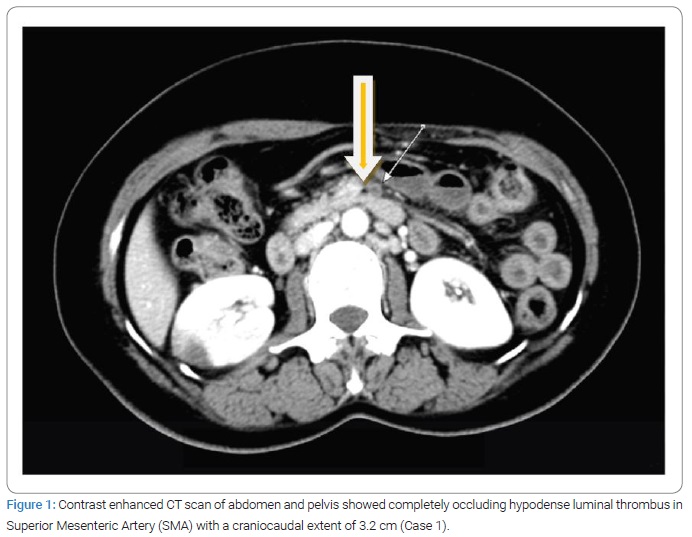
On evaluation, a contrast-enhanced CT scan of the abdomen and pelvis showed a completely occluding hypodense luminal thrombus in Superior Mesenteric Artery (SMA) with a craniocaudal extent of 3.2 cm (Figure 1), along with thickened bowel wall and abnormal enhancement, suggesting bowel wall ischemia. There was no previous history of any kind of thromboembolic event (TEE). Symptoms had started on the 15th day of the first cisplatin administration, while radiological documentation of TEE was done on the 16th day. Discontinuous partial occluding thrombus in descending thoracic aorta with bilateral renal infarct was also noted. CT Angiography revealed long segment thrombosis of SMA. She underwent emergency laparotomy, which showed viable bowel; hence bowel resection was not done. An ileostomy was performed to monitor ischemic bowel changes at the stoma level.
Post exploratory laparotomy, heparin infusion was continued at 800 IU/ml. She had no improvement in abdominal pain despite heparin infusion, and with viable bowel stoma, endovascular revascularization was planned. Percutaneous mechanical aspiration was unsuccessful. Intraarterial local thrombolysis was started with an injection of urokinase, but infusion had to be discontinued due to hemodynamically instability. She was then managed with continuous heparin infusion. Her stoma showed signs of infarction three days later, and CT showed no wall enhancement of small bowel loops. Emergency exploratory laparotomy was performed, gangrenous distal jejunum, entire ileum, caecum, and proximal ascending colon resected, and side to anastomosis between the jejunum and transverse colon was done. Unfortunately, the post-surgery patient had an anastomotic leak. Re-exploration and jejunostomy were advised, but the patient and her family refused the procedure. Best supportive care was given, but three weeks later, the patient succumbed and expired due to leak complications and peritonitis.
Case Presentation 2
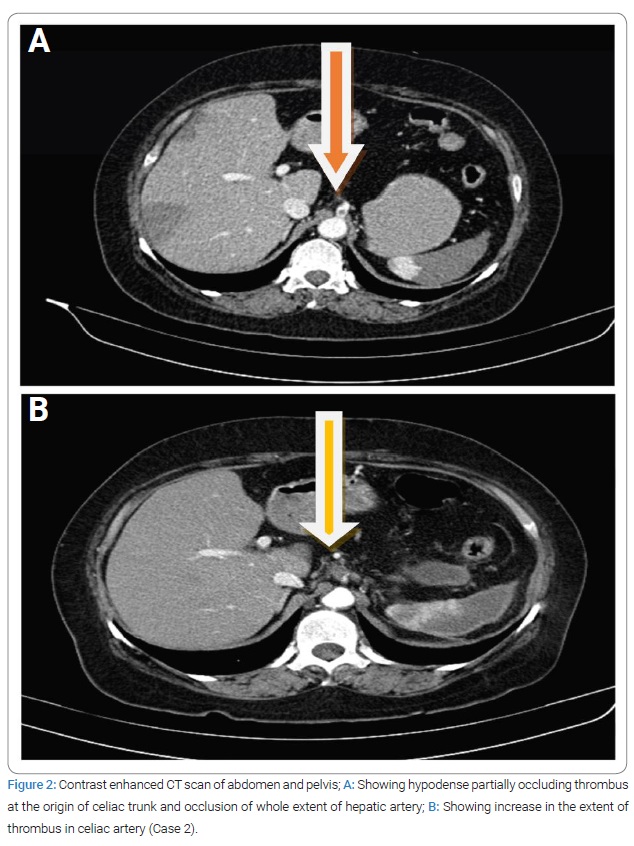
A 55-year-old lady with no comorbidities and addictions with no past or family history of any TEE/predisposing disorders was diagnosed with cervical cancer, FIGO stage IIB. She had three full-term normal vaginal deliveries with no significant events. She was planned for definitive chemo-radiotherapy. During the treatment period, after the completion of 14 fractions of radiotherapy (to the pelvis only) and three cycles of cisplatin (weekly 40 mg/m2), the patient presented to the emergency department with complaints of severe abdominal pain. On evaluation with contrast-enhanced CT scan of abdomen and pelvis showed hypodense partially occluding thrombus at the origin of the celiac trunk and occlusion of the whole extent of the hepatic artery (Figure 2A), with associated infarcts in both liver lobes. Symptoms had started on the 19th day of the first cisplatin initiation, while radiological documentation of TEE was done on the 21st day. Later CT angiogram was done, which showed another thrombus in a terminal branch of the splenic artery, resulting in a whole splenic infarct. She has managed with enoxaparin 60 mg twice a day. She completed external beam radiotherapy to a total dose of 50 y/25# to the pelvis, followed by three fractions of intracavitary brachytherapy. Further concurrent chemotherapy was not given post thrombosis episode. There was no previous history of any kind of thromboembolic event (TEE).
On follow-up at six weeks post completion of radio-chemotherapy, there was an increase in the extent of thrombus in the celiac artery, as seen in (Figure 2B). The enoxaparin dose was increased to 80 mg twice a day. Clinically patient was asymptomatic, and at three months of follow-up imaging, no change in the extent of thrombus was observed. A reduced dose of enoxaparin was continued for six months and imaging at this time showed no thrombus and normal celiac trunk and its branches. Presently patient is clinically controlled and on regular follow-up.
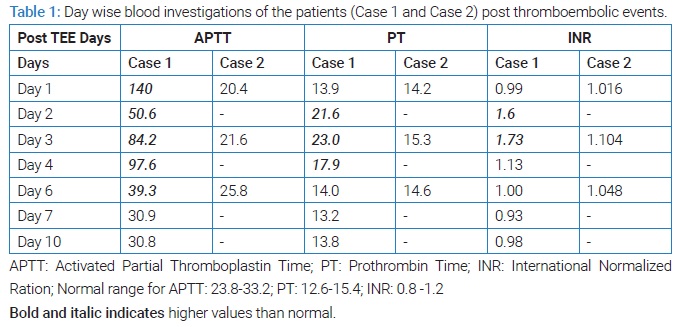
In these both cases, we found TEE can have a spectrum of ‘No symptoms’ (2nd Case) to ‘Life-threatening events’ (1st Case), which warranted emergency laparotomy and followed by many complications including gangrene, anastomotic leak, and peritonitis leading to death. The corresponding important investigations are mentioned in (Table 1). Timely diagnosis of TEE and intervention is the key to avoiding non-oncological events/ deaths in cancer patients.
Discussion
Concurrent chemo-radiotherapy is a cornerstone in the management of locally advanced cervical cancer through stages Ib2 to IVa. In cervical malignancy, the reported incidence of thromboembolic events in literature is 11.7% [6]. Though TEE events have been reported in the literature for various malignancies, TEE in cervical malignancy is not commonly reported, while it (especially arterial thromboembolism) is more commonly associated with primary lung and kidney [7]. The total annual number of cervical cases reported in our institute is 1000. So, occurring in 2 cases out of these explains the event’s rarity.
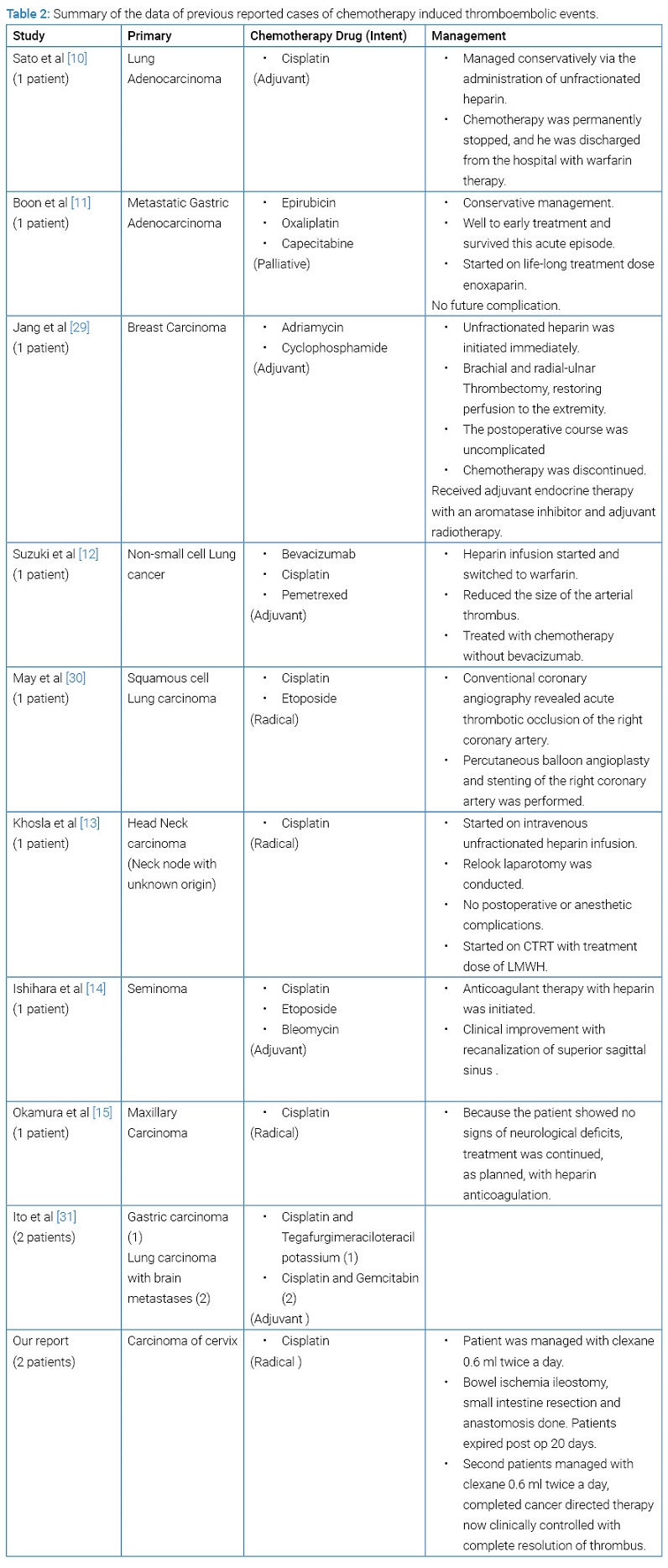
In both patients of this report, TEE occurred during initial cycles of chemotherapy, matching the available data in the literature [8], which explains Cisplatin as the causative factor. Various chemotherapeutic agents increase the risk of thromboembolic events in patients with malignancy [4] (Table 2), and Cisplatin is one of the most common chemotherapeutic agents associated with TEE [9]. There are few cases reported wherein Cisplatin was the triggering factor [10–15], including our cases; no other risk factors could be identified. The reported mechanisms by which Cisplatin triggers vascular events are endothelial damage, the elevation of plasma levels of the von-Willebrand factor, reactive thrombocytosis, and altered balance between thrombosis and dissolution of blood clots also conditions like drug-induced reduction of left ventricular function [4,9,16]. Cisplatin can cause direct endovascular damage by free radical-induced lipid peroxidation in endothelial cells. This can cause intimal thickening and platelet aggregation [17]. The use of Cisplatin in certain malignancies is known to produce a hypercoagulable state, increasing the thrombosis risk [18]. Apart from this, the possible reasons behind endothelial dysfunction are cancer-related hypercoagulability and radiotherapy leading to arterial or venous thromboembolism [19]. The other risk factors for TEE include age, obesity, hyperlipidemia, smoking, hypercoagulable states like malignancy, dehydration in addition to hereditary causes. Brachytherapy has also been reported as a risk feature for TEE, which is crucial in the management of cervical cancer [20], which was not yet part of treatment in our patients as TEE occurred during the external beam radiotherapy phase only.
In the thrombus formation process, fibrin and extracellular DNA complexed with histone proteins form the outer scaffold, which may be important in determining thrombus susceptibility to Tissue Plasminogen Activator (TPA) and thrombolysis. The proportion of proteins is, in part, determined by the ratio of the endothelial cell surface to blood volume. Large vessels (GIT vessels) have a less cell surface to blood volume ratio that favors procoagulants. As the ratio of procoagulants to anticoagulants increases, the risk of thrombus formation also increases. That’s the possible reason behind the thrombus being formed in GIT blood vessels in both of the cases [21].
It has been shown in studies that thromboembolic events are associated with poor survival outcomes [22]. Thrombosis, associated with cancers apart from affecting survival, also increases financial expenditure. Henrik et al. have reported that in the group with cancer at the time of venous thromboembolism, the one-year survival rate was 12 percent, as compared with 36 percent in the control group (P < 0.001), and the mortality ratio for the entire follow-up period was 2.20 (95 percent confidence interval, 2.05 to 2.40) [22].
LMWHs are preferred in post-surgical settings in cancer in view of a good safety profile and reliability. They are associated with increased quality of life as a primary/secondary prophylaxis. But they are not recommended as routine prophylaxis in advanced cancer patients receiving chemotherapy (LOE II, Grade B) or in ambulatory cancer patients receiving adjuvant chemotherapy or hormone therapy [23].
As many of the patients receive Cisplatin (Platinum-based chemotherapy), we cannot really start giving prophylaxis to everyone because of these uncommon events. Recently, risk assessment models (RAMs) have been developed specifically for TEE risk in cancer patients, which include factors like age, race and immobility status, primary site, stage of cancer, whether or not the patient is receiving chemotherapy, biomarkers such as platelet count, tissue factor or D-dimer levels [24,25]. American Society of Hematology 2021 guidelines propose various pharmacologic options for TEE treatment and prevention in cancer patients, including Unfractionated Heparin (UFH), Low-Molecular-Weight Heparins (LMWHs), fondaparinux (an indirect synthetic inhibitor of activated factor Xa), Vitamin K Antagonists (VKAs), and Direct Oral Anticoagulants (DOACs) including direct thrombin inhibitors and direct factor Xa inhibitors. Thromboprophylaxis is not recommended for ambulatory patients receiving chemotherapy at low risk of VTE [26]. Low-Molecular-Weight Heparin (LMWH) as an initial treatment who developed VTE, LMWH or fondaparinux for surgical patients with cancer, DOAC in ambulatory patients with cancer receiving systemic therapy at high risk of VTE, or for the long-term treatment of VTE [26]. Treatment or prophylaxis of VTE for patients with cancer must always balance the risk of recurrent VTE events with the increased risk of anticoagulant-related bleeding.
As we have discussed earlier, Cisplatin has a significantly greater risk of inducing thromboembolic events when compared to other platinum-based chemotherapeutic agents [27]. There are many investigational radiosensitizers that are being used in the place of Cisplatin (or along with Cisplatin) in various phases of clinical trials, which include carboplatin, cetuximab, gemcitabine, capecitabine, topotecan, none of which found out to be superior to Cisplatin (CDDP) [28]. The uncommon risk of developing TEE due to Cisplatin is not enough to remove Cisplatin as the first choice of radiosensitizer along with radiation in cervical cancer treatment in radical or adjuvant chemoradiation setting.
Arterial thrombosis has a dismal prognosis if not diagnosed early. Prompt diagnosis and anticoagulation initiation are important to prevent thrombus propagation and complications. The treating physician should have a high level of clinical suspicion for Cisplatin-induced vascular complications. Relevant imaging must be done even when subtle symptoms are present to prevent complications. The principal goals for these patients are improving survival, symptoms, and quality of life.
Along with cancer-directed therapy, it is equally important to treat other comorbidities like Diabetes, Hypertension, etc. Lifestyle modification plays an important role in the management of these comorbidities. This incidence reporting will help in the future to better understand the magnitude of this problem, as currently, there is no consensus on using thromboprophylaxis in patients receiving chemotherapy.
Conclusion
Arterial and venous thromboembolism can lead to fatal complications. Therefore, prompt diagnosis and early intervention are needed. Though rare in carcinoma cervix, arterial thromboembolism should be kept in mind during patient evaluation, especially for those who are receiving thrombogenic chemotherapy. Cancer patients who are at high risk of TEE should be identified, and appropriate measures should be taken before starting chemotherapy. Aggressive management can improve a patient’s survival.
Acknowledgments
Author Contributions: All authors provided critical feedback, discussed and commented on the manuscript.
Patient consent: We have taken the informed consent from the patients.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
Keywords
Arterial thrombosis; Cervical cancer; Cisplatin; Radiotherapy
Cite this article
Sneha R, Nilesh R, Roshan P, Lavanya G, Jaya G, Ashwin D, et al. Arterial thrombosis in cervical cancer patients treated with cisplatin based concurrent radiation-chemotherapy: a case report. Clin Oncol J. 2022;3(2):1–7.
Copyright
© 2022 Gurram Lavanya. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).




