A Typical Progression Pattern after Pre-Docetaxel Abiraterone for Metastatic Prostate Cancer: Report of Two Cases
* Fernandez Alfonso;
Lee Benjamin R;
Gonzalez Abraham;
Avila-Monteverde E;
Chipollini Juan;
Pautler SE;
-
* Fernandez Alfonso: Division of Urology, San Jose Hospital Oncology Center, Hermosillo 83270, Mexico.
-
Lee Benjamin R: Department of Urology, University of Arizona College of Medicine, AZ 85724, United States.
-
Gonzalez Abraham: Department of Pathology, CIMA Hospital, Hermosillo 83280, Mexico.
-
Avila-Monteverde E: Division of Oncology, San Jose Hospital Oncology Center, Hermosillo 83270, Mexico.
-
Chipollini Juan: Division of Urology, San Jose Hospital Oncology Center, Hermosillo 83270, Mexico.
-
Pautler SE: Department of Urology, St. Joseph’s Hospital, London, ON N6A 4V2, Canada.
-
Sep 19, 2022 |
-
Volume: 3 |
-
Issue: 1 |
-
Views: 2358 |
-
Downloads: 1620 |
Abstract
After starting treatment with Abiraterone acetate, we report two patients with advanced prostate cancer with rapid progression and metastasis into the urinary bladder. Treatment was pursued with palliative intent.
Introduction
Prostate cancer is the most common non-cutaneous cancer in men in the United States. 160,000 men were diagnosed with prostate cancer in 2017 [1]. However, concerns regarding the presence of indolent disease and the side effects and risks associated with diagnostic procedures and treatment have generated controversy about screening and early detection [1]. In patients with metastatic prostate cancer (MPc), androgen deprivation therapy (ADT) is the standard of care; although, most patients will progress to castration-resistant disease (CRPC) within approximately one year [2].
The condition that leads to death in the majority of MPc patients is precisely the development of castration resistance [3]. In addition, abiraterone acetate is a selective, irreversible inhibitor of CYP17, an enzyme critical in producing androgens in the testes, adrenal glands, and prostate cancer tissue [4].
Inhibiting CYP17 in combination with ADT results in more effective androgen depletion induced by surgical castration or by GnRH analogs alone [4].
In patients with metastatic hormone-sensitive disease, Abiraterone has been demonstrated to be as effective as Docetaxel in reducing the risk of death while reducing the risk of disease progression and improved quality of life [5]. And also increases radiographic progression-free survival when compared to ADT plus placebo [4,6,7]. Furthermore, in patients with metastatic CRPC, Abiraterone has increased overall survival [3,8]. However, many patients with MPc will eventually develop local symptoms due to disease progression.
Bladder tumors that are metastatic in origin have been reported ranging from 14% to 33%, with the most common primary sites being the colon, rectum, and cervix [9]. In a series by Bates et al., 4.4% of all bladder tumors were metastatic, and only 0.8% of the total came from a prostatic adenocarcinoma [9,10]. Here, we report two patients with MPc treated with first-line Abiraterone/Prednisone/Leuprolide that quickly experienced treatment failure with recurrence to the bladder. To our knowledge, there are no cases of this rapid pattern of progression after initiation of Abiraterone.
Case Presentation
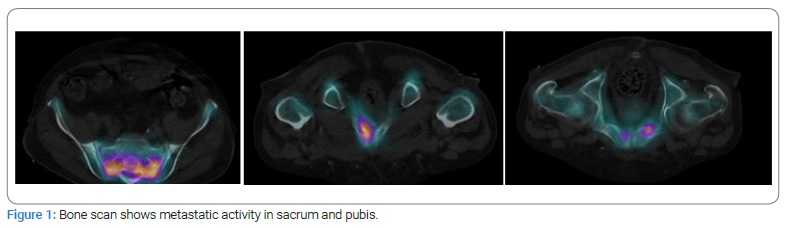
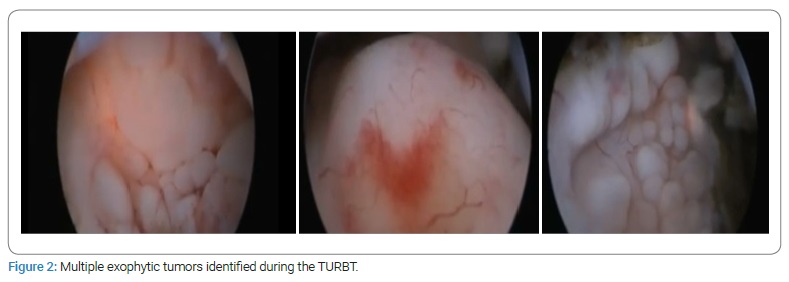
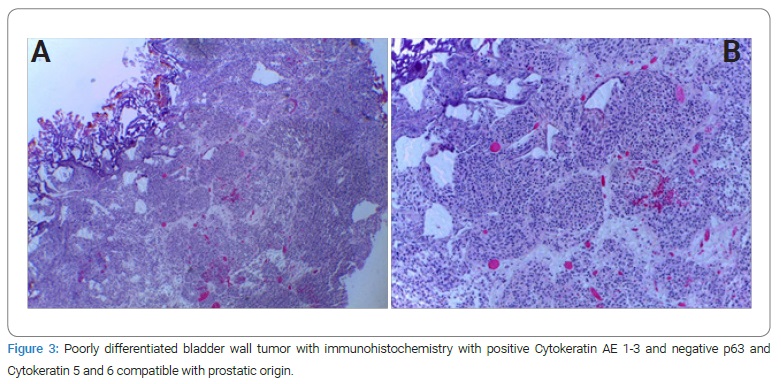
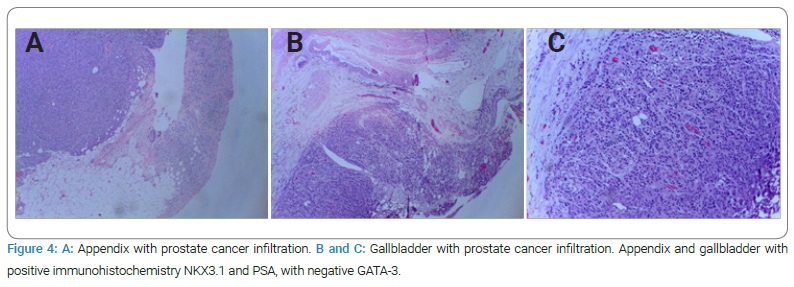
Case I: A 69-year-old male patient with a history of diabetes and hypertension. Two years prior, he was diagnosed with Gleason 10 (5+5) in 12 of 12 cores, with perineural infiltration. A bone scan showed multiple metastases, including pubis and sacrum (Figure 1). He was treated with tri-monthly Gosereline (10.8 mg) with Bicalutamide 50 mg PO every 24 hours for 18 months. Unfortunately, he developed castration-resistant disease. Since Enzalutamide was not yet approved, he was started on Abiraterone 250 mg, four tablets PO every 24 hours with Prednisone 10 mg PO every 24 hours, and was switched to Leuprolide 22.5 mg tri-monthly because of insurance and availability. Eight months into his treatment and after an initial PSA response, he had an episode of gross hematuria with clots. A bladder ultrasound revealed a bladder mass and underwent transurethral resection of bladder tumor (TURBT). Various exophytic masses were observed arising from the bladder neck into the bladder’s lumen (Figure 2). With Pathology reporting a poorly differentiated malignancy with immunohistochemistry withCK AE 1-3+, P63 -, CK 5/6 -, compatible with a prostatic origin; a re-TURBT came back with the same results (Figure 3). He declined chemotherapy, and forty-eight months after his initial diagnosis, he developed gastrointestinal bleeding. He underwent right hemicolectomy and cholecystectomy because of neoplastic lesions suggestive of metastases that were confirmed histologically (Figure 4), dying soon after the procedure. His last PSA was measured at 2.2 ng/ml.
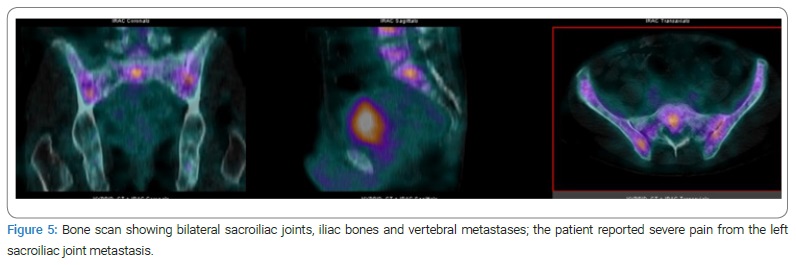
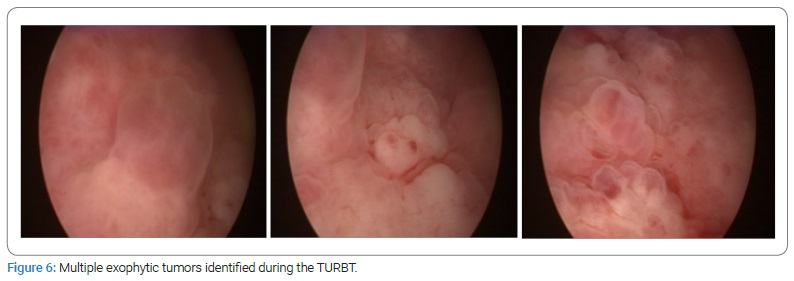
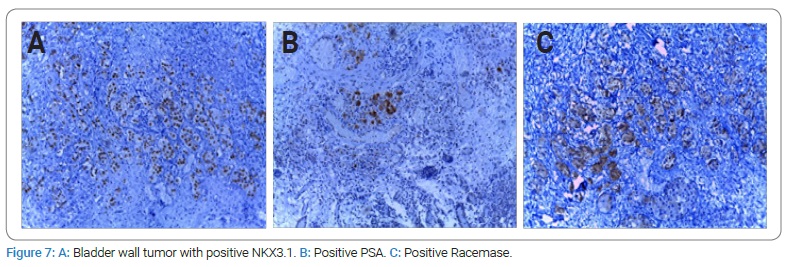
Case II: 58-year-old male patient with a family history of prostate cancer and elevated PSA who underwent prostate biopsy and was diagnosed with Gleason 8 (4+4), in 90% of the tissue, with perineural infiltration and a PSA of 14.3 ng/ml. He presented with acute urinary retention and a bone scan with multiple metastases in vertebrae, bilateral sacroiliac joints, and iliac bones; the left sacroiliac joint metastasis was symptomatic (Figure 5). He was started on initial antiandrogen therapy with Bicalutamide 50 mg PO every 24 hours 10 days prior to initiation of 45 mg Leuprolide injection. Next, he started Abiraterone 250 four tablets PO every 24 hours with Prednisone 10 mg PO every 24 hours. He also had Denosumab for fracture prevention due to vertebral metastases. Two months later, his PSA came down to 1.1 ng/ml, referring to less bone pain and a major improvement in his general health status. However, four months into his treatment with Abiraterone, he developed gross hematuria with clots. A TURBT was performed, identifying multiple exophytic bullae suggesting direct extension from the bladder neck into the trigone (Figure 6); the pathologist’s report came back with infiltration by a poorly differentiated carcinoma consistent with primary prostate adenocarcinoma with positive immunohistochemistry for NKX 3.1, PSA, and Racemase (Figure 7). The Abiraterone was discontinued after almost six months, and he received seven cycles of Docetaxel and Prednisone, dying ten months after starting Abiraterone.
Discussion
Metastatic prostate cancer has been reported to have a dismal 5-year survival rate of 29.3% [11]. In recent years, the survival in metastatic patients has been more clearly defined due to the improved therapeutic options for these patients. The castration-sensitive state appears to be diverse with both androgen receptor-positive and negative cells, by which ADT eventually selects a clone resistant to conventional castration (CRPC). The most common sites for MPc are bone (84%), lymph nodes (10.6%), liver (10.2%) and thorax (9.1%) [12]. In a population of Docetaxel-naïve patients treated with either first-line Abiraterone or Enzalutamide for metastatic CRPC, an increased proportion of post-treatment lymph node, bone, and liver. Metastases were identified, suggesting that CRPC is being converted to a clinically aggressive disease in some patients [13].
Abiraterone acetate has shown a survival benefit in the metastatic setting, either before or after treatment with docetaxel. Furthermore, since MPc is a heterogeneous disease, there has been a paradigm change in its therapeutic approaches, with phase III studies confirming a survival advantage with either chemohormonal therapy or Abiraterone [14]. Thus, there is strong evidence to support the role of multi-agent therapy at the start of ADT.
Due to improved survival for men with newly diagnosed MPc, many patients will eventually develop symptomatic local progression due to an untreated primary. To the best of our knowledge, the discovery of local prostate cancer recurrence in the bladder after initiation of Abiraterone is not well described in the current literature. Thus far, the question of whether providing a palliative procedure to improve patients’ quality of life outweigh the inherent risks of a major operation remains largely unanswered.
Although patient No. 1 did not have immunohistochemistry tested for PSA in his first bladder tumor sample, the rest of the immunohistochemistry is highly suggestive of a prostatic origin [15]. In addition, the fact that later the patient developed metastases to the appendix and gallbladder confirms this finding.
Conclusion
Prostate cancer presenting in the bladder after starting Abiraterone represents an uncommon metastatic spread with few treatment options. In addition, this unusual tumor spread appears to herald more aggressive tumor biology.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Litwin MS, Tan HJ. The diagnosis and treatment of prostate cancer: a review. JAMA. 2017;317(24):2532–2542.
- Chi KN, Protheroe A, Rodriguez-Antolin A, Facchini G, Suttman H, Matsubara N, et al. Patient-reported outcomes following abiraterone acetate plus prednisone added to androgen deprivation therapy in patients with newly diagnosed metastatic castration-naïve prostate cancer (LATITUDE): an international, randomised phase 3 trial. Lancet Oncol. 2018;19(2):194–206.
- Biró K, Budai B, Szonyi M, Küronya Z, Gyergyay F, Nagyiványi K, et al. Abiraterone acetate + prednisolone treatment beyond prostate specific antigen and radiographic progression in metastatic castration-resistant prostate cancer patients. Urol Oncol. 2018;36(2):81.e1–81.e7.
- James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Mes. 2017;377(4):338–51.
- Feyerabend S, Saad F, Li T, Ito T, Diels J, Van Sanden S, et al. Survival benefit, disease progression and quality-of-life outcomes of Abiraterone acetate plus prednisone versus docetaxel in metastatic hormone-sensitive prostate cancer: A network meta-analysis. Eur J Cancer. 2018;103:78–87.
- Rydzewska LHM, Burdett S, Vale CL, Clarke NW, Fizazi K, Kheoh T, et al. Adding Abiraterone to androgen deprivation therapy in men with metastatic hormone-sensitive prostate cancer: A systematic review and meta-analysis. Eur J Cancer. 2017;84:88–101.
- Fizazi K, Tran NP, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377(4):352–360.
- Oh WK, Cheng WY, Miao R, Vekeman F, Gauthier-loiselle M, Duh MS, et al. Real-world outcomes in patients with metastatic castration-resistant prostate cancer receiving second-line chemotherapy versus an alternative androgen receptor-targeted agent (ARTA) following early progression on a first-line ARTA in a US community oncology setting. Urol Oncol. 2018;36(11):500.e1–500.e9.
- Bates AW, Baithun SI. Secondary neoplasms of the bladder are histological mimics of nontransitional cell primary tumours: clinicopathological and histological features of 282 cases. Histopathology. 2000;36(1):32–40.
- Hallemeier CL, Kohli M, Chandan VS, Miller RC, Choo R. Multiple urinary bladder masses from metastatic prostate adenocarcinoma. Rare Tumors. 2010;2(4):e65.
- National Cancer Institute. Cancer Stat Facts: Prostate Cancer. USA: NIH; 2018.
- Gandaglia G, Abdollah F, Schiffmann J, Trudeau V, Shariat SF, Kim SP, et al. Distribution of metastatic sites in patients with prostate cancer: A population-based analysis. Prostate. 2014;74(2):210–216.
- Lee EK, Teply BA, Maughan BL, Carcucci MA, Antonarakis ES, Denmeade SR. Patterns of metastatic disease progression after treatment with first-line enzalutamide or Abiraterone in castration-resistant prostate cancer (CRPC). J Clin Oncol. 2016;34(15):e16539.
- Damodaran S, Kyriakopoulos CE, Jarrard DF. Newly Diagnosed Metastatic Prostate Cancer: Has the Paradigm Changed? Urol Clin North Am. 2017;44(4):611–621.
- Molinié V, Baumert H. [New markers in prostate biopsies]. Actas Urol Esp. 2007;31(9):1009–1024.
Keywords
Pre-Docetaxel abiraterone; Metastatic prostate cancer; Urinary bladder
Cite this article
Fernandez A, Benjamin, Abraham G, Avila-Monteverde E, Chipollini J, Pautler SE. A typical progression pattern after pre-docetaxel abiraterone for metastatic prostate cancer: report of two cases. Clin Oncol J. 2022;3(1):1–5.
Copyright
© 2022 Fernandez Alfonso. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).







