Triple Negative Breast Cancer in Togolese Women: Clinicopathological Features and Outcomes
* Adani-Ife AA;
Diakite A;
Doh K;
Darre T;
Amegbor K;
-
* Adani-Ife AA: Department of Oncology, University Teaching Hospital of Lomé, Togo.
-
Diakite A: Radiotherapy Center of Lomé, Togo.
-
Doh K: Department of Pathology, University Teaching Hospital of Lomé, Togo.
-
Darre T: Department of Pathology, University Teaching Hospital of Lomé, Togo.
-
Amegbor K: Department of Pathology, University Teaching Hospital of Lomé, Togo.
-
May 06, 2022 |
-
Volume: 3 |
-
Issue: 1 |
-
Views: 2060 |
-
Downloads: 1816 |
Abstract
Background: Clinicopathological features in Triple Negative Breast Cancer may be different by ethnicity. However, little is known about the characteristics of this subtype in Togolese women. The purpose of this retrospective study was to analyze the clinical and pathological features of triple negative breast cancer in Togolese women and evaluate the survival of patients treated.
Methods: Medical records of all breast cancer patients diagnosed between 2016 and 2021 were reviewed and triple negative breast cancers were selected for the study. Clinicopathologic variables and clinical outcomes of these patients were evaluated.
Results: Sixty-two breast cancer cases (19.1%) were identified as triple negative breast cancer. The mean age of patients was 53.1 years with a range from 30 to 85 years. Ten patients (16.1%) were below 40 years of age and forty-six patients (74.2%) were menopausal. The majority of patients (n = 56, 90.3%) had infiltrating ductal carcinoma. More than half of the patients (n = 32, 51.6%) had histological grade 3 tumors. Most patients (n = 37, 59.7%) presented with stage III and IV disease. A modified radical mastectomy was done in forty-two patients (80.8%) with non-metastatic disease. Fifty-one patients (82.3%) were treated with anthracyclines and taxane-based chemotherapy. Nineteen patients (36.5%) received radiotherapy in an adjuvant setting. The median follow-up time was 33.8 months (range from 1 month to 72 months). Sixteen patients had a recurrence and 28 deaths occurred. The overall survival at 5 years of patients with non-metastatic and metastatic disease was respectively 65.4% and 20%.
Conclusion: Triple negative breast cancer occurred in predominance in post-menopausal Togolese women. Poor survival in our patients was correlated with larger tumor size and the presence of metastasis at diagnosis. This emphasizes the importance of early detection of breast cancer in our country.
Abbreviations
TNBC: Triple Negative Breast Cancer; ER: Estrogen Receptor; PR: Progesterone Receptor; HER2: Human Epidermal growth factor Receptor 2; BRCA: Breast Cancer gene; WHO: World Health Organization; AJCC: American Joint Committee on Cancer; TNM: Tumor Node Metastases; SPSS: Statistical Package for the Social Sciences.
Introduction
Triple Negative Breast Cancer (TNBC) accounts for 10% to 20% of all breast cancer worldwide but remains the deadliest subgroup of breast cancer [1,2].
TNBC is defined by the absence of expression of the Estrogen Receptor (ER), Progesterone Receptor (PR), and Human Epidermal Growth Factor Receptor 2 (HER2) over expression [3]. TNBC is a highly heterogeneous form of breast cancer that is often characterized by aggressive clinicopathologic features [4,5]. Patients with TNBC are more likely to experience relapse within the first 3 years and are at high risk of death in the first five years following diagnosis [5,6].
Many reports from different countries across the world documented clinicopathological features in TNBC that may be different by ethnicity [7–10]. Population-based studies have reported that West African ancestry is associated with a higher TNBC prevalence and worse disease outcomes [11]. In Togo, a small country in West Africa, previous studies showed that breast cancer is common cancer among women and triple negative breast cancer has been reported as the predominant molecular subtype [12–15]. However, little is known about the characteristics of this subtype in Togolese women.
The purpose of this retrospective study, carry out for the first time in Togo was to analyze the clinical and pathological features of triple negative breast cancer in Togolese women and evaluate the survival of patients treated.
Patients and Methods
Patients
This is a retrospective and descriptive study of the patients who attended the oncology department of the Sylvanus Olympio teaching hospital of Lome for a confirmed diagnosis of breast cancer from March 2016 to March 2021 (5 years). The medical files of these patients were reviewed.
The status of ER, PR, and HER2 was determined by immunohistochemical staining for quantitative and qualitative assessment. Negativity was defined for less than 1% positive tumors cells. HER 2-neu score ≤ was considered negative.
The data collected include clinical variables such as age at diagnosis, laterality, body mass index, family history of cancer, and parity. Variables related to tumor characteristics (including tumor size, histological tumor type, tumor grade, lymph node involvement, stage, BRCA mutation), treatment modalities and survival of patients were also collected.
Breast cancer has been classified according to the World Health Organization 2012 (WHO 2012). Histological classification was performed using the Nottingham classification system and staging according to the 8th edition of the AJCC classification of 2017.
Follow-up of patients was scheduled to be every 3 months for the first two years post-treatment, every 6 months for the next 3 years, and then annually. Survival data were updated through medical records and Telephone interviews. Our final follow-up deadline was March 01, 2022.
Statistic
Statistical analysis and data processing was performed with the software SPSS version 20.
Descriptive data were expressed in percentage or median or mean.
The overall survival was defined as the time from the date of diagnosis (date of pathological confirmation of breast cancer) to the date of death or last follow-up. Survival estimations were performed using the Kaplan -Meier method. Analysis of possible factors affecting the survival was done. Univariate and multivariate analyses were performed using a Cox proportional hazard regression model for survival. All the variables with a p-value at or below 0.2 in the univariate analysis were included in the multivariate analysis. A p-value of < 0.05 was considered statistically significant
Ethics
This study was approved by the “Comite de Bioethique pour la Recherche en Sante (CBRS)” (Bioethics Committee for Health Research) from the Togo Ministry of Health, Ref N0: 0101/2016/MS/CAB/DGS/DPLET/CBRS. Because this study was a retrospective analysis, our request for the waiver of informed consent was approved.
Results
Clinicopathological features
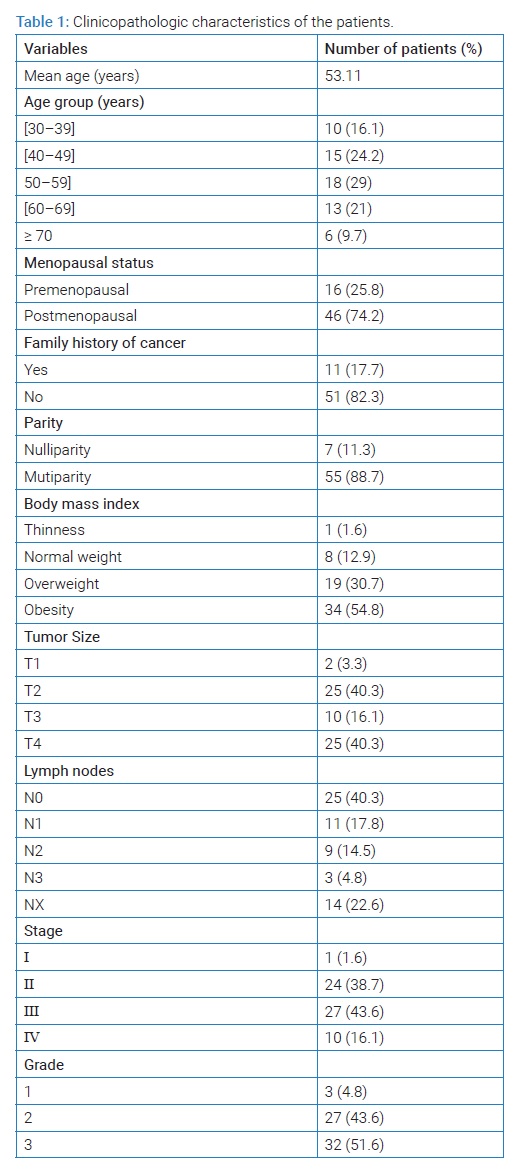
Among 325 patients diagnosed with breast cancer, 62 (19.1%) were identified as having triple negative breast cancer. The mean age of patients was 53.1 years with a range from 30 years to 85 years. One-sixth of patients (n = 10, 16.1%) were below 40 years of age. Forty-six patients (74.2%) were menopausal and seven patients (11.3%) were nulliparous. Eleven patients (17.7%) had a family history of cancer. In the Body Mass Index evaluation, nineteen patients (30.7%) were overweight and obesity was found in more than half of the patients (n = 34, 54.8%).
The tumor was right-sided in 28 patients (45.2%) and left-sided in 34 patients (54.8%) at presentation. The majority of patients (n = 56, 90.3%) had an infiltrating ductal carcinoma, infiltrating lobular carcinoma represented 3%. One medullary carcinoma and three mucinous carcinomas were diagnosed. More than half of the patients (n = 32, 51.6%) had histological grade 3 tumors. For the lymph node involvement, 37.1% of patients (n = 23) had positive Lymph nodes. According to TNM classification, 25 patients (40.3%) presented with T4 tumors whereas only two patients (3.3%) had T1 tumors. At diagnosis, only one patient (1.6%) had stage I, 24 patients (38.7%) had stage II, 27 patients (43.6%) had stage III, and 10 patients (16.1%) presented with metastatic disease.
The identification of BRCA mutation was performed in only one patient who did not have mutation genes. Clinicopathologic characteristics of patients are shown in (Table 1).
Treatment details and outcomes
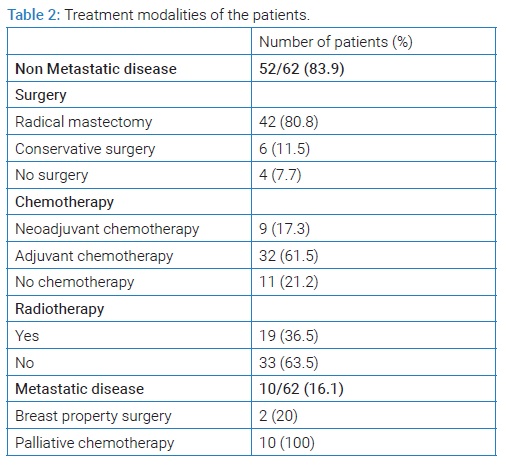
Among the 52 patients with non-metastatic disease (stage I, II, III), 48 patients (92.3%) underwent surgery. A modified radical mastectomy was done in 42 patients (80.8%) and 6 patients (11.5%) underwent breast conservative surgery.
Forty-one patients were treated with anthracyclines and taxane-based chemotherapy (41/52, 78.8%). Nine patients (9/41, 22%) received neoadjuvant chemotherapy and adjuvant chemotherapy was done in 32 patients (32/41, 78%). Nineteen patients (19/52, 36.5%) received radiotherapy in an adjuvant setting.
The ten patients who presented with metastatic disease at diagnosis received palliative chemotherapy. All of them were treated in the first line by an anthracyclines regimen. Two underwent property breast surgery. (Table 2) summarizes the treatment details of all patients.
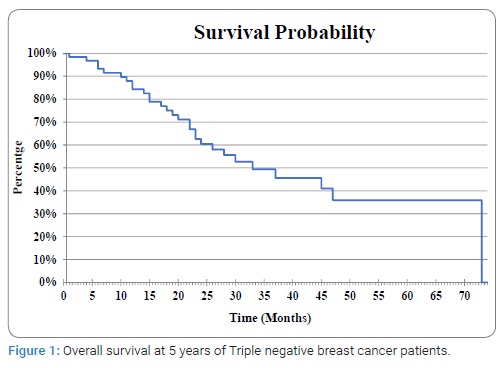
The median follow-up time was 33.8 months [range from 1 month to 72 months]. During the follow-up, 16 patients experienced recurrence, and 28 deaths occurred. One patient presented local recurrence and distal organ involvement was observed in 15 patients. Lung (8/15), liver (8/15), and nodes (6/15) was the most common site of distal recurrence. Three patients had brain recurrence and bone recurrence was observed in two patients. The overall survival at 5 years of all the patients was 34.7% (Figure 1). The overall survival at 5 years of patients with non-metastatic (stage I–III) and metastatic disease (stage IV) was respectively 65.4% and 20%. The association between clinicopathologic variables and overall survival was analyzed.
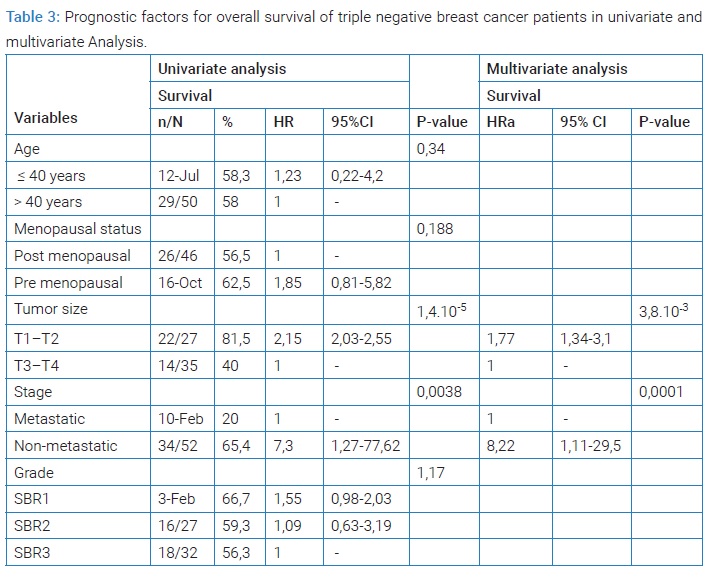
The results of univariate and multivariate analyses are summarized in (Table 3). Larger tumor size (T3–T4) and metastatic stage at diagnosis were significantly correlated with shorter overall survival. No Statistical difference was found in survival according to the age, the menopausal status, and tumor grade.
Discussion
This study described the clinical profile, pathological characteristics, and outcomes of Togolese patients with TNBC. Several studies have suggested that women of African ancestry in the United States and continental Africa have higher frequencies of TNBC [1,16,17]. However, in our study, TNBC accounted for 19.1% of breast cancer which is consistent with data reported worldwide [1,2]. Previous studies reported a younger age at diagnosis in TNBC [2,5]. In the current study, the mean age of patients was 53.1 years which is similar to that reported by Dent in Western populations but higher than that observed in studies from Koweit and Morocco [6,18–20].
In our series, sixteen percent of patients were below the age of forty years, Thike also reported similar findings in the Asian population from Singapore [21].
Like In Algeria and India, the majority of our patients were menopausal women. This is not concordant with many reports in the literature which described TNBC in earlier onset often before menopause [9,19,22–24].
There are several risk factors for TNBC such as BRCA mutation and Body Mass Index (BMI). Indeed, obesity has been linked to increasing the risk of developing TNBC in women of all ethnic backgrounds and the association between BRCA mutations and the development of TNBC is well established [25,26]. Obesity was observed in more than half of our patients (54.8%). We found 17.7% of family history of breast cancer but BRCA mutation research was performed on only one patient due to its high cost in our setting.
At diagnosis, TNBC is commonly for larger tumor sizes [6]. In our study, more than half of the patients had T3–T4 tumors and the rates of stage III and metastatic cancer are high (59.7%). This can be explained by the rapid growth of Triple Negative tumors, the delay in presentation often observed in our patients and other sub-Saharan countries [14,17,27], and the lack of an organized national screening program for breast cancer in our country.
Triple negative carcinomas are mainly high-grade ductal carcinomas [2,4,5]. In our study, similar to many others [19,21,28], infiltrating ductal carcinoma was the most frequent histological type and most tumors (51.6%) were of high grade.
Metastatic progression in TNBC is characterized by the predominance of visceral sites (liver and brain) and a lower incidence of bone metastasis [29,30]. In this study, we observed a high rate of visceral metastasis in the liver and lung but only three patients presented brain metastasis. This is contrary to data reported by Singh in India [23], where the brain was the most common site of recurrence followed by the liver and lung. However, our data corroborated the lower incidence of bone metastasis in TNBC as these metastases were found in only two patients.
When TBNC is diagnosed earlier and treated adequately, the survival rates are comparable to non-TNBC [31].
Patients with the non-metastatic disease are managed with surgical intervention followed by adjuvant systemic chemotherapy and radiotherapy (when indicated) to reduce the risk of recurrence; neoadjuvant chemotherapy is considered in patients with the non-operable disease [32]. In addition, as recently published [33], Olaparib, a Poly (adenosine diphosphate–ribose) polymerase inhibitor can be used as adjuvant therapy for patients with BRCA1 or BRCA2 germline mutation associated with early triple negative breast cancer who have a high risk of recurrence despite standard-of-care local and systemic therapy.
The type of surgical procedure depends on the extent of the initial disease. In this study majority of the patients were in stage III and mastectomy was the main surgical intervention. A similar finding has been reported in Indian studies where most TNBC presented with stage III [23,34,35]. However, most of our patients (78%) underwent upfront surgical treatment followed by adjuvant systemic chemotherapy. Only nine patients (22%) were considered for neoadjuvant chemotherapy.
Treatment of metastatic TNBC is highly based on systemic chemotherapy. Nevertheless, some targeted drugs were recently approved for these patients such as two poly (ADP-ribose) polymerase inhibitors (olaparib and talazoparib) in patients with germline BRCA1 BRCA2 mutations; immune checkpoint inhibitors (atezolizumab and pembrolizumab) in association with chemotherapy if programmed death-ligand 1 positive; and an antibody-drug conjugate (sacituzumab- govitecan) in heavily pretreated patients [36].
Access to all these drugs is extremely limited in our practice because of their high cost and technical difficulties to determine individuals that would ideally benefit from them. Metastatic patients in our study were exclusively treated with chemotherapy.
In this study, the survival rate of TNBC patients at 5 years was 34.7%. This rate is lower than that reported in several studies in Africa [19,20,22]. However, the patients with non-metastatic disease had longer survival than those diagnosed with metastatic disease (65.4% vs. 20%). Variable survival outcomes of TNBC have been reported around the world with different prognosis factors [5,17,19,23,28]. The stage at diagnosis is a well-known prognosis factor in breast cancer as was confirmed in this study. Like in Indians and Chinese patients [23,28], tumor size was also correlated to survival in our patients. The advanced stage at diagnosis in most of our patients is due to late presentation, socio-economic constraints, and limited access to health care. In our results, no statistical difference was found in survival according to age, menopausal status, and tumor grade. This can be due to the small size of our study population. Further studies with a larger sample size can help to understand the effects of the factors on survival.
The present study has a limitation of selection bias which may be due to retrospective nature, non-availability of immunochemistry status in all breast cancer received during the study period, and the absence of fluorescence In situ hybridization analysis in patients with HER2 score 2.
Conclusion
Triple negative breast cancer occurred in predominance in post-menopausal Togolese women. However, this study shows that most of the clinical and pathological features in our patients are in accordance with the previous finding in the literature such as high grade, advanced stage at diagnosis, and poor prognosis. Survival in our patients was correlated with larger tumor size and the presence of metastasis at diagnosis. This emphasizes the importance of early detection of breast cancer in our country and to improve the access to treatment.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Wright N, Rida P, Rakha E, Agboola A, Aneja R. Panoptic overview of triple-negative breast cancer in Nigeria: Current challenges and promising global initiatives. J Glob Oncol. 2018;4:1–20.
- Kumar P, Aggarwal R. An overview of triple-negative breast cancer. Arch Gynecol Obstet. 2016;293(2):247–269.
- Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98(19):10869–10874.
- Oakman C, Viale G, Di Leo A. Management of triple negative breast cancer. Breast. 2010;19(5):312–321.
- Rhee J, Han SW, Oh DY, Kim JH, Im SA, Han W, et al. The clinicopathologic characteristics and prognostic significance of triple-negativity in node-negative breast cancer. BMC Cancer. 2008;8:307.
- Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–4434.
- Fejerman L, Haiman CA, Reich D, Tandon A, Deo RC, John EM, et al. An admixture scan in 1,484 African American women with breast cancer. Cancer Epidemiol Biomarkers Prev. 2009;18(11):3110–3117.
- Palmer JR, Ruiz-Narvaez EA, Rotimi CN, Cupples LA, Cozier YC, Adams-Campbell LL, et al. Genetic susceptibility loci for subtypes of breast cancer in an African American population. Cancer Epidemiol Biomarkers Prev. 2013;22(1):127–134.
- Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the carolina breast cancer study. JAMA. 2006;295(21):2492–2502.
- Swede H, Gregorio DI, Tannenbaum SH, Brockmeyer JA, Ambrosone C, Wilson LL, et al. Prevalence and prognostic role of triple-negative breast cancer by race: a surveillance study. Clin Breast Cancer. 2011;11(5):332–341.
- Jiagge E, Jibril AS, Chitale D, Bensenhaver JM, Awuah B, Hoenerhoff M, et al. Comparative analysis of breast cancer phenotypes in African American, White American, and West versus East African patients: Correlation between African ancestry and triple-negative breast cancer. Ann Surg Oncol. 2016;23(12):3843–3849.
- Amégbor K, Darré T, Ayéna KD, Padaro E, Tengué K, Abalo A, et al. Cancers in Togo from 1984 to 2008: epidemiological and pathological aspects of 5251 cases. J Cancer Epidemiol. 2011;2011:319872.
- Darré T, Kpatcha TM, Bagny A, Maneh N, Gnandi-Piou F, Tchangai B, et al. Descriptive epidemiology of cancers in Togo from 2009 to 2016. Asian Pac J Cancer Prev. 2017;18(12):3407–3411.
- Adani-Ifè AA. Practice of Oncology in Low- income setting: A review of four years activities in togo. Asian Pac J Cancer Care. 2021;6(3):289–296.
- Adani-Ifè AA, Amégbor K, Doh K, Darré T. Breast cancer in togolese women: immunohistochemistry subtypes. BMC Women’s Health. 2020;20(1):261.
- Proctor E, Kidwell KM, Jiagge E, Bensenhaver J, Awuah B, Kofi G, et al. Characterizing breast cancer in a population with increased prevalence of triple-negative breast cancer: Androgen receptor and ALDH1 expression in Ghanaian women. Ann Surg Oncol. 2015;22(12):3831–3835.
- Der EM, Gyasi RK, Tettey Y, Edusei L, Bayor MT, Jiagge E, et al. Triple-negative breast cancer in Ghanaian women: The Korle Bu teaching hospital experience. Breast J. 2015;21(6):627–633.
- Fayaz MS, El-Sherify MS, El-Basmy A, Zlouf SA, Nazmy N, George T, et al. Clinicopathological features and prognosis of triple negative breast cancer in Kuwait: A comparative/perspective analysis. Rep Pract Oncol Radiother. 2014;1 9(3):173–181.
- Derkaoui T, Bakkach J, Mansouri M, Loudiyi A, Fihri M, Alaoui FZ, et al. Triple negative breast cancer in North of Morocco: clinicopathologic and prognostic features. BMC Women’s Health. 2016;16(1):68.
- Rais G, Raissouni S, Aitelhaj M, Rais F, Naciri S, Khoyaali S, et al. Triple negative breast cancer in Moroccan women: clinicopathological and therapeutic study at the National Institute of Oncology. BMC Womens Health. 2012;12:35.
- Thike AA, Cheok PY, Jara-Lazaro AR, Tan B, Tan P, Tan PH. Triple-negative breast cancer: clinicopathological characteristics and relationship with basal-like breast cancer. Mod Pathol. 2010;23(1):123–133.
- Haddad S, Frimeche Z, Lakehal A, Sifi K, Satta D, Abadi N. Descriptive study of triple negative breast cancer in Eastern Algeria. Pan Afr Med J. 2018;29:45.
- Singh D, Roy N, Das SM. Epidemiology, pattern of recurrence and survival in triple-negative breast cancer: a retrospective analysis. Asian Pac J Cancer Care. 2020;5(2):87–94.
- Al jarroudi O, Abda N, Brahmi SA, Afqir S. Triple negative breast cancer at the university hospital mohammed VI – oujda. Asian Pac J Cancer Prev. 2017;18(1):195–200.
- Bandera EV, Maskarinec G, Romieu I, John EM. Racial and ethnic disparities in the impact of obesity on breast cancer risk and survival: A global perspective. Adv Nutr. 2015;6(6):803–819.
- Schneider BP, Winer EP, Foulkes WD, Garber J, Perou CM, Richardson A, et al. Triple-negative breast cancer: risk factors to potential targets. Clin Cancer Res. 2008;14(24):8010–8018.
- Nwagu GC, Bhattarai S, Swahn M, Ahmed S, Aneja R. Prevalence and mortality of triple-negative breast cancer in West Africa: biologic and sociocultural factors. J Glob Oncol. 2021;7:1129–1140.
- Yuan N, Meng M, Liu C, Feng L, Hou L, Ning Q, et al. Clinical characteristics and prognostic analysis of triple–negative breast cancer patients. Mol Clin Oncol. 2014;2(2):245–251.
- Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat. 2009;115(2):423–438.
- Beca F, Santos R, Vieira D, Zeferino L, Dufloth R, Schmitt F. Primary relapse site pattern in women with triple-negative breast cancer. Pathol Res Pract. 2014;210(9):571–575.
- Schwentner L, Wolters R, Koretz K, Wischnewsky MB, Kreienberg R, Rottscholl R, et al. Triple-negative breast cancer: The impact of guideline-adherent adjuvant treatment on survival--a retrospective multi-centre cohort study. Breast Cancer Res Treat. 2012;132(3):1073–1080.
- Bergin ART, Loi S. Triple-negative breast cancer: recent treatment advances [version 1; peer review: 2 approved]. F1000Research. 2019;8(F1000 Faculty Rev):1342.
- Tutt ANJ, Garber JE, Kaufman B, Viale G, Fumagalli D, Rastogi P, et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. 2021;384(25):2394–2405.
- Lakshmaiah KC, Das U, Suresh TM, Lokanatha D, Babu GK, Jacob LA, et al. A study of triple-negative breast cancer at a tertiary cancer care center in Southern India. Ann Med Health Sci Res. 2014;4(6):933–937.
- Chandra D, Suresh P, Sinha R, Azam S, Batra U, Talwar V, et al. Eight year survival analysis of patients with triple negative breast cancer in India. Asian Pac J Cancer Prev. 2016;17(6):2995–2999.
- Cipriano E, Mesquita A. Emerging therapeutic drugs in metastatic triple-negative breast cancer. Breast Cancer (Auckl). 2021;15:11782234211002491.
Keywords
Triple negative; Breast cancer; Epidemiology; Survival; Togo
Cite this article
Adani-Ife AA, Diakite A, Doh K, Darre T, Amegbor K. Triple negative breast cancer in togolese women: clinicopathological features and outcomes. Clin Oncol J. 2022;3(1):1–6.
Copyright
© 2022 Adani-Ife AA. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).




