Implementation of Photobiomodulation for Radio or Chemo-Induced Mucositis
* Lamyaa Nkhali;
Billiard-Sandu C;
Al-Halabi A;
Bensadoun RJ;
-
* Lamyaa Nkhali: Oreliance Health Center, Avenue Jacqueline Auriol, Saran, France.
-
Billiard-Sandu C: Gustave Roussy Cancer Campus, Rue Edouard Vaillant, Villejuif, France.
-
Al-Halabi A: Oreliance Health Center, Avenue Jacqueline Auriol, Saran, France.
-
Bensadoun RJ: High Energy Center, Bd Pasteur, Nice, France.
-
Mar 23, 2022 |
-
Volume: 3 |
-
Issue: 1 |
-
Views: 8084 |
-
Downloads: 2729 |
Abstract
Aim: Photobiomodulation (PBM) therapy is used for the management of oral mucositis in patients treated with radiotherapy and/or chemotherapy. The aim of this study is to evaluate the efficacy and safety of PBM for oral mucositis during radiotherapy with or without chemotherapy.
Materials and Methods: A total of 62 patients diagnosed with a mucositis grade 2 or more and managed with PBM were evaluated. Clinical response was assessed by the physician daily, using the Common Terminology Criteria for Adverse Events (CTCAE) v 4.0 2010 and by patient using a questionnaire before and at the end of the treatment.
Results: All patients accepted PBM when proposed; 79% were men and 21% women. Sixty-nine percent of patients had oropharyngeal cancer and 60% had a stage IV a disease. Average age was 64 years. Chemotherapy was used in 72% of patients and 84% of them had Cisplatin. Patients had a grade 2 mucositis for 82%, grade 3 for 16% and grade 4 for 2%. The median area was 3 cm². Median digital pain scale was 6 before LLLT and 2 after treatment, 61% had strong opioids before PBM and only 10% at the end. Seventy-four percent of patients had a weight loss more than 5% and 53% of patients were fed by nasogastric tube or gastrostomy (before or during radiotherapy). Only 1 patient had a radio chemotherapy interruption because of deterioration of general condition. A loco-regional recurrence occurred in 26% of patients and 45% of patients had no progression at time of evaluation.
Conclusion: PBM treatment seems to be a well-tolerated, easy to use and safe treatment in oral mucositis. Its analgesic effect is interesting.
Introduction
PBM is defined as a “mechanism, by which non-ionizing optical radiation in the spectral range of the visible or near infrared is absorbed by endogenous chromophores to trigger photo physical and photochemical events without causing thermal damage, resulting in physiological changes and therapeutic benefits” [1].
Its wavelength ranges from red light (400 nm to 700 nm) to infrared (700 nm to 1100 nm). The therapeutic dose is defined by the density measured in Joules (J/cm2) and the energy is calculated by E = P × t where E is the energy expressed in Joules (J) or kWh, P is the power in Watts or kW and t the duration of use in seconds or hours.
The wave of red or infrared light is absorbed by the mitochondria. This results in stimulation of cytochrome C, an increase in the production of Adenosine Triphosphate (ATP) and of Nitric Oxide (NO) with the consequence of an improvement of tissue perfusion, as well as in cell growth factors [2]. Finally, PBM, reduces nociception secondary to the elimination of mediators of acute inflammation and increased axonal activity [3].
Mucositis is described as erythema and painful ulcerative lesions of the oral mucosa observed in cancer patients treated with chemotherapy and/or radiotherapy [4].
Mucositis is a common side effect of chemotherapy and/or radiation therapy that causes pain, difficulty eating, salivation and eating disorders, oral ulcerations and even discontinuation of treatments. Its etiopathological origin is complex and multifactorial. Treatment is mainly symptomatic with pain control. The objective of this work is to evaluate the efficacy and safety of PBM in patients with radio- and/or chemo-induced mucositis within our institution since its implementation in January 2016.
Materials and Methods
Patients
We studied the records of patients treated with PBM between January 2016 and October 2020 for mucositis in patients treated with radiotherapy with or without chemotherapy.
A total of 62 patients were treated for at least grade 2 mucositis. The data concerned the age, gender, grade and surface of the mucositis, tumor site, treatment (radiotherapy with or without chemotherapy), severity of pain, nutrition and recurrence.
Photobiomodulation protocol
The equipment used is a Hilaris® 150 mW diode laser with a wavelength of 660 nm. Application is done daily after each radiation therapy session to all grade 2 mucositis lesions at least. The application is painless, athermic, odorless and completely silent. The patient wears glasses for retinal protection. The operator also wears glasses for his protection but nevertheless allowing observing the beam and its limits.
Application is done at a distance ≤ 15 mm from the mucosa sweeping all areas of grade 2 mucositis at least. The energy dose delivered is 4 J/cm2. The optical fiber is held perpendicular to the surface of the mucosa during application.
The duration of the treatment of an area will be determined by a corresponding abacus: [t(s) = energy (J/cm2) × surface (cm2) / power (W)]. Application times will be respected using a timer. The overall duration will be a few minutes, varying according to the size of the surface to be treated. Necessary analgesic treatments and common oral care are also prescribed.
Evaluation
Before each session, the physician collected the data entered in the Mosaiq® software. A simple anatomical diagram was also made with drawing of the surface to be treated. Clinical response was performed daily by the physician prior to each PBM session using the Common Terminology Criteria for Adverse Events (CTCAE) v4.0 2010 classification (Appendix 1) and by the patient using a questionnaire before and at the end of treatment (Appendix 2).
Pain was assessed by a visual analogue scale scoring ranging from 0 to 10.
Appendix 1
Common Terminology Criteria for Adverse Events (CTCAE) v 4.0 2010.
It includes separate subjective and objective scales for mucositis:
- Grade 1—Erythema of the mucosa.
- Grade 2—Patchy ulcerations or pseudomembranes.
- Grade 3—Confluent ulcerations or pseudomembranes; bleeding with minor trauma.
- Grade 4—Tissue necrosis; significant spontaneous bleeding; life-threatening consequences.
- Grade 5—Death.
Appendix 2
Patient questionnaire at the beginning and end of PBM sessions:
- Do you have oral pain?
- Can you quantify it from 0 (no pain) to 10?
- Do you take pain treatments? If so, which ones? Cortisone? Do you make mouthwashes?
- What is your current weight? And your usual weight?
- Can you eat properly?
Results
PBM was accepted by all patients who had the indication, 62 patients between January 2016 and October 2020.
Patients were mostly men, 79% compared to 21% of women. The most common site was the oropharynx in 69% of cases and 60% had advanced stage IV a cancer. The average age was 64 years (Table 1).
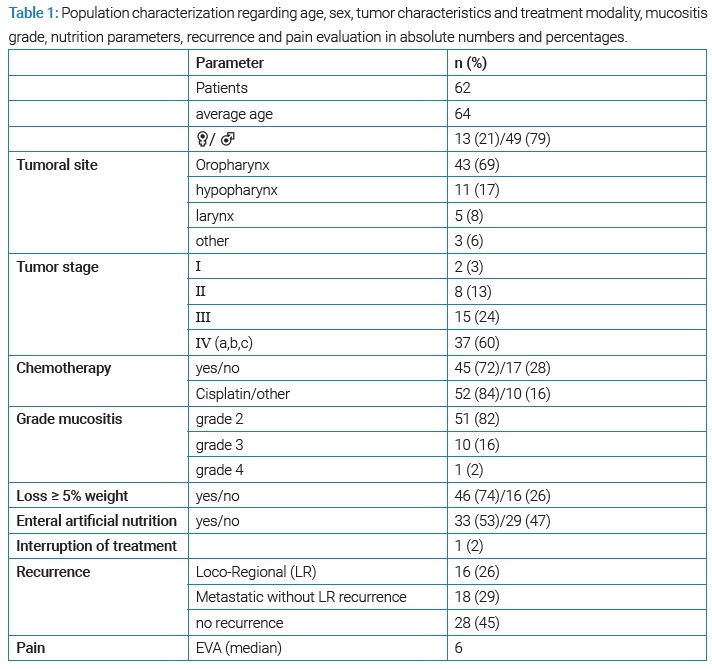
Radiotherapy protocol was with volumetric modulated arc therapy from 60 Gy to 70 Gy. Patients receiving chemotherapy were 72%, protocol of 100 mg/m2 cisplatin every 3 weeks was the most common in 84% of cases.
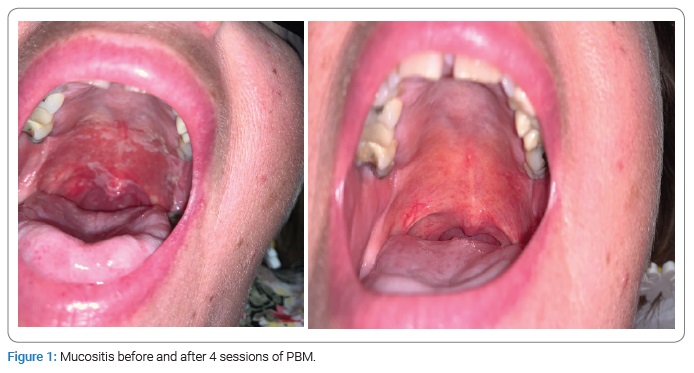
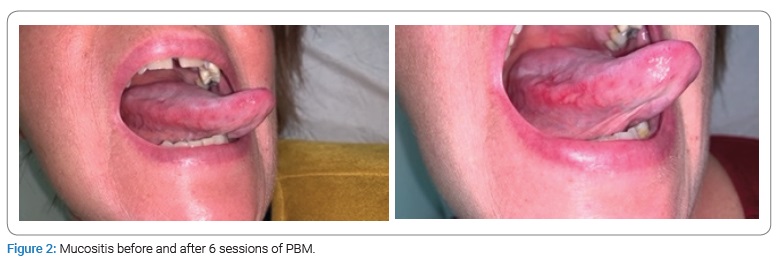
Before having PBM, patients had grade 2 mucositis in 82% of cases, grade 3 in 16% and grade 4 in 2%. PBM was carried out daily, and with an average number of sessions of 8, 94% of patients had a grade 1 mucositis at the end (Figure 1 and Figure 2). The median area of mucositis was 3 cm2.
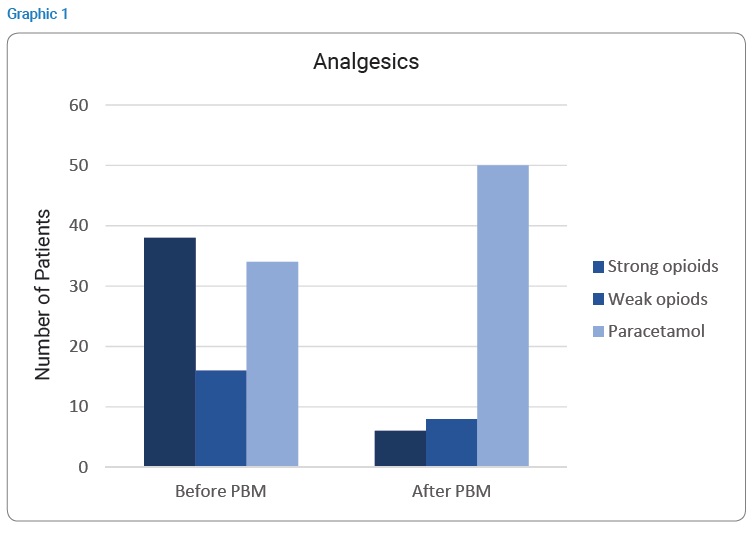
The median pain score was 6 before PBM treatment and 2 at the end of treatment. We evaluated analgesic levels before and immediately after PBM. If 61% of patients had strong opiods, they were only 10% after. Also, 26% had weak opiods before and only a half after (Graphic 1).
We systematically looked for parameters of malnutrition and in particular a loss of more than 5% of body weight before or during treatment. Seventy-four percent of the patients were malnourished and 53% of them were fed enterally (nasogastric tube or gastrostomy).
Only 1 patient had an interruption of treatment (radio chemotherapy) due to an alteration in the general condition. A loco-regional recurrence was diagnosed in 26% of patients and 45% of patients had no recurrence at the time of data collection.
Discussion
Mucositis is frequent and its consequences are not negligible, ranging from impaired quality of life of the patient (pain) to food discomfort, weight loss or even hospitalization and interruption of treatments that can have consequences on healing.
In the majority of studies, this complication occurs in up to 80% of patients receiving high-dose chemotherapy, and up to 100% of patients receiving radiation therapy for head and neck cancer, and about 20% to 40% in those receiving conventional chemotherapy [4–5].
A review of the literature reporting 33 randomised trials including patients treated with radiotherapy with or without chemotherapy found severe grade 3 or 4 mucositis in 34% of patients and 11% of patients had an interruption or modification of their radiotherapy protocol [6].
PBM is interesting to prevent or treat mucositis, it is experiencing a growing boom in oncology. The Ottaviani 2013 study showed clinical improvement in mucositis from day 5 of treatment in patients receiving chemotherapy [7]. After 3 weeks of treatment, 70% of patients had no pain or mucositis.
In our study, the median number of sessions was 6. During or after the PBM the majority of patients no longer had mucositis and stopped analgesics, even opioids. Our protocols were cautious during the initiation of the technique in our center, but have since been modified, the sessions are continued with excellent efficiency and safety, allowing complete relief of the patient.
Several studies have used PBM, both preventive and therapeutic. The results showed the superiority of the prophylactic (preventive) application of PBM over the therapeutic approach [8–9]. The interest of preventive treatment was found in a review of the recent literature [10].
Preventive PBM was selected as a recommendation and no longer only as a suggestion in the MASCC guidelines updated in 2020 for patients treated with high-dose chemotherapy, for patients receiving radiotherapy for head and neck cancer with or without chemotherapy. Suggestions for dosimetric protocols were also made [11].
Dosimetry
Despite ample evidence of the effectiveness of PBM, its place is still under discussion in therapeutic management, particularly because of the heterogeneity of protocols and lack of dosimetric data.
The review by Zadik et al. [12] established recommendations for PBM protocols in the prevention of oral mucositis in patients treated with hematology (transplantation), radiotherapy or radio chemotherapy for head and neck cancer. Two main protocols stood out: for red light (633 nm to 685 nm) and the other for infrared light (780 nm to 830 nm).
However, there is a lack of standardization in the prevention of oral mucositis in patients treated with chemotherapy and radio chemotherapy.
Zecha et al. [13] proposed protocols for mucositis in patients treated for head and neck cancer or chemotherapy for bone marrow transplantation as a preventive and curative. The optimal protocol is as follows: wavelength, usually between 633 nm and 685 nm or 780 nm to 830 nm; energy at the output of the laser or Light-Emitting Diode (LED) between 10 mW and 150 mW; dose of 2 J (J/cm(2)) to 3 J (J/cm(2)), and not more than 6 J/cm(2) on the treated tissue surface; treatment two to three times a week until daily; type of pulsed emission (< 100 Hz); and intraoral and/or transcutaneous route of administration.
Local recurrence
In our study, we did not find recurrence during PBM or during treatment even in patients without previous surgery. However, the tumor is always avoided by the beam as a precaution.
Results on the proliferative effect of PBM cancer cells are controversial in vitro and in vivo [14].
Cells pre-exposed to PBM radiation still seem to have different responses depending on whether they are healthy or cancerous [15].
Finally, the literature review published by Bensadoun RJ et al. [16] did not find any demonstrated deleterious effects of PBM in vivo and human studies. PBM is still to be used with caution and avoid on the tumor.
Clinical practice in radiotherapy
PBM brings excellent results on the prevention and treatment of mucositis. It could be used in clinical trials of radiotherapy in particular those which are toxic by their fractionation or acceleration but also the combination of systemic treatments. Indeed, studies have found excellent carcinological results, but the main limit found was toxicity and in particular mucositis [17–18].
Finally, PBM becomes an essential support that avoids complications and reduces the cost of treatment (hospitalizations, drug prescriptions, etc.) [19]. It has become an essential support in our practice.
Conclusion
In our experience, PBM treatment is simple to set up, effective and appears safe. Patients are relieved and it allows them to continue treatments in the best conditions.
The treatment is currently recommended as a preventive which will prevent the appearance of severe mucositis. However, the different machines, protocols and dosimetric parameters remain to be homogenized.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Anders JJ, Arany PR, Baxter GD, Lanzafame RJ. Light-emitting diode therapy and low-level light therapy are photobiomodulation therapy. Photobiomodul Photomed Laser Surg. 2019;37(2):63–65.
- De Freitas LF, Hamblin MR. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron. 2016;22(3):7000417.
- Chow RT, Armati PJ. Photobiomodulation: implications for anesthesia and pain relief. Photomed Laser Surg. 2016;34(12):599–609.
- Lalla RV, Bowen J, Barasch A, Elting L, Epstein J, Keefe DM, et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2014;120(10):1453–1461.
- Kashiwazaki H, Matsushita T, Sugita J, Shigematsu A, Kasashi K, Yamazaki Y, et al. Professional oral health care reduces oral mucositis and febrile neutropenia in patients treated with allogeneic bone marrow transplantation. Support Care Cancer. 2012;20(2):367–373.
- Trotti A, Bellm LA, Epstein JB, Frame D, Fuchs HJ, Gwede CK, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66(3):253–262.
- Ottaviani G, Gobbo M, Sturnega M, Martinelli V, Mano M, Zanconati F, et al. Effect of class IV laser therapy on chemotherapy-induced oral mucositis: a clinical and experimental study. Am J Pathol. 2013;183(6):1747–1757.
- Carvalho P a. G, Jaguar GC, Pellizzon AC, Prado JD, Lopes RN, Alves FA. Evaluation of low-level laser therapy in the prevention and treatment of radiation-induced mucositis: a double-blind randomized study in head and neck cancer patients. Oral Oncol. 2011;47(12):1176–1181.
- Arora H, Pai KM, Maiya A, Vidyasagar MS, Rajeev A. Efficacy of He-Ne Laser in the prevention and treatment of radiotherapy-induced oral mucositis in oral cancer patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(2):180–186, 186.e1.
- Cronshaw M, Parker S, Anagnostaki E, Mylona V, Lynch E, Grootveld M. Photobiomodulation and Oral Mucositis: A Systematic Review. Dent J (Basel). 2020;8(3):87.
- Elad S, Cheng KKF, Lalla RV, Yarom N, Hong C, Logan RM, et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2020;126(19):4423–4431.
- Zadik Y, Arany PR, Fregnani ER, Bossi P, Antunes HS, Bensadoun RJ, et al. Systematic review of photobiomodulation for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer. 2019;27(10):3969–3683.
- Zecha JAEM, Raber-Durlacher JE, Nair RG, Epstein JB, Elad S, Hamblin MR, et al. Low-level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: part 2: proposed applications and treatment protocols. Support Care Cancer. 2016;24(6):2793–2805.
- De Pauli Paglioni M, Araújo ALD, Arboleda LPA, Palmier NR, Fonsêca JM, Gomes-Silva W, et al. Tumor safety and side effects of photobiomodulation therapy used for prevention and management of cancer treatment toxicities. A systematic review. Oral Oncol. 2019;93:21–28.
- Barasch A, Raber-Durlacher J, Epstein JB, Carroll J. Effects of pre-radiation exposure to LLLT of normal and malignant cells. Support Care Cancer. 2016;24(6):2497–2501.
- Bensadoun RJ, Epstein JB, Nair RG, Barasch A, Raber-Durlacher JE, Migliorati C, et al. Safety and efficacy of photobiomodulation therapy in oncology: A systematic review. Cancer Med. 2020;9(22):8279–8300.
- Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32(27):2940–2950.
- Bensadoun RJ, Etienne MC, Dassonville O, Chauvel P, Pivot X, Marcy PY, et al. Concomitant B.I.D. radiotherapy and chemotherapy with cisplatin and 5-fluorouracil in unresectable squamous-cell carcinoma of the pharynx: clinical and pharmacological data of a French multicenter phase II study. Int J Radiat Oncol Biol Phys. 1998;42(2):237–45.
- Rodrigues-Oliveira L, Kowalski LP, Santos M, Marta GN, Bensadoun RJ, Martins MD, et al. Direct costs associated with the management of mucositis: A systematic review. Oral Oncol. 2021;118:105296.
Keywords
Photobiomodulation; Oral mucositis; Radiotherapy; Chemotherapy
Cite this article
Nkhali L, Billiard-Sandu C, Al-Halabi A, Bensadoun RJ. Implementation of photobiomodulation for radio or chemo-induced mucositis. Clin Oncol J. 2022;3(1):1–6.
Copyright
© 2022 Lamyaa Nkhali. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).




