Equilibrative Nucleoside Transporter 1 Expression is a Prognostic Marker in Pancreatic Adenocarcinoma
* Bruno Nervi;
Sergio Vargas-Salas;
Juan-Carlos Roa;
Nicolás Jarufe;
Sebastián Mondaca;
Marcelo Garrido;
Héctor Galindo;
José Peña;
Cesar Sanchez;
Jorge Madrid;
Carolina Ibañez;
M. Loreto Bravo;
Nathaly de la Jara;
Patricia Macanas-Pirard;
Richard Broekhuizen;
Eduardo Viñuela;
Sabrina Muñiz;
Gloria Aguayo;
Maeva del Pozo;
Pablo Ramírez;
Carlos Cárcamo;
Mauricio Mahave;
Manuel Meneses;
Jean M Butte;
Jorge Gajardo;
Valentina Hornig;
Marianella Balocchi;
Mauricio P. Pinto;
-
* Bruno Nervi: Department of Hematology and Oncology, Pontifical Catholic University of Chile, Santiago, Chile; Millennium Institute on Immunology and Immunotherapy, Pontifical Catholic University of Chile, Santiago, Chile.
-
Sergio Vargas-Salas: Department of Hematology and Oncology, Pontifical Catholic University of Chile, Santiago, Chile.
-
Juan-Carlos Roa: Department of Pathology, Pontifical Catholic University of Chile, Santiago, Chile; Department of Digestive Surgery, Pontifical Catholic University of Chile, Santiago, Chile.
-
Nicolás Jarufe: Department of Digestive Surgery, Pontifical Catholic University of Chile, Santiago, Chile.
-
Sebastián Mondaca: Department of Hematology and Oncology, Pontifical Catholic University of Chile, Santiago, Chile.
-
Marcelo Garrido: Department of Hematology and Oncology, Pontifical Catholic University of Chile, Santiago, Chile.
-
Héctor Galindo: Department of Hematology and Oncology, Pontifical Catholic University of Chile, Santiago, Chile.
-
José Peña: Department of Hematology and Oncology, Pontifical Catholic University of Chile, Santiago, Chile.
-
Cesar Sanchez: Department of Hematology and Oncology, Pontifical Catholic University of Chile, Santiago, Chile.
-
Jorge Madrid: Department of Hematology and Oncology, Pontifical Catholic University of Chile, Santiago, Chile.
-
Carolina Ibañez: Department of Hematology and Oncology, Pontifical Catholic University of Chile, Santiago, Chile.
-
M. Loreto Bravo: Department of Hematology and Oncology, Pontifical Catholic University of Chile, Santiago, Chile.
-
Nathaly de la Jara: Department of Pathology, Pontifical Catholic University of Chile, Santiago, Chile.
-
Patricia Macanas-Pirard: Department of Hematology and Oncology, Pontifical Catholic University of Chile, Santiago, Chile.
-
Richard Broekhuizen: Department of Hematology and Oncology, Pontifical Catholic University of Chile, Santiago, Chile.
-
Eduardo Viñuela: Department of Digestive Surgery, “Dr. Sótero del Río” Hospital, Santiago, Chile.
-
Sabrina Muñiz: Department of Digestive Surgery, “Dr. Sótero del Río” Hospital, Santiago, Chile.
-
Gloria Aguayo: Department of Pathology, “Dr. Sótero del Río: Hospital, Santiago, Chile.
-
Maeva del Pozo: Institute of Medicine, Austral University of Chile, Valdivia, Chile.
-
Pablo Ramírez: Institute of Medicine, Austral University of Chile, Valdivia, Chile.
-
Carlos Cárcamo: Institute of Medicine, Austral University of Chile, Valdivia, Chile.
-
Mauricio Mahave: Institute for Oncology,“Fundación Arturo López Pérez”, Santiago, Chile.
-
Manuel Meneses: Institute for Oncology,“Fundación Arturo López Pérez”, Santiago, Chile.
-
Jean M Butte: Institute for Oncology,“Fundación Arturo López Pérez”, Santiago, Chile.
-
Jorge Gajardo: Institute for Oncology,“Fundación Arturo López Pérez”, Santiago, Chile.
-
Valentina Hornig: Department of Hematology and Oncology, Pontifical Catholic University of Chile, Santiago, Chile.
-
Marianella Balocchi: Department of Hematology and Oncology, Pontifical Catholic University of Chile, Santiago, Chile.
-
Mauricio P. Pinto: Department of Hematology and Oncology, Pontifical Catholic University of Chile, Santiago, Chile.
-
Dec 30, 2021 |
-
Volume: 2 |
-
Issue: 2 |
-
Views: 2579 |
-
Downloads: 2410 |
Abstract
Pancreatic Adenocarcinomas (PACs) are highly aggressive neoplasms. To date, surgery remains the most effective treatment. Adjuvant Gemcitabine is commonly is used as a chemotherapeutic for resected PAC patients. Previous studies demonstrate that Gemcitabine is incorporated into cancer cells via the Equilibrative Nucleoside Transporter 1 (ENT1). Hence, ENT1 expression has been proposed as a predictive biomarker in PAC. However, evidence supporting its use as a prognostic biomarker is still lacking. Here, we investigated the prognostic value of ENT1 in resected chemotherapy naïve PAC patients. A total of 87 patients with resected PAC were retrospectively included (exploratory cohort). ENT1 expression was analyzed by tissue microarray and categorized as high (H-ENT1) or low (L-ENT1). Clinical-pathological characteristics analyzed survival rates. Our findings were validated in an independent, consecutively collected cohort of 50 patients.
ENT1 expression was higher in tumors with favorable prognostic factors and correlated with overall survival. ENT1 expression showed to be a better prognostic factor compared to pathological characteristics. H-ENT1 determined a higher risk of mortality in pT1–T2 (HR 2.32) and pN0 (HR 2.34) in our exploratory and validation cohorts. Finally, in a Cox proportional hazard backward-model, L-ENT1 correlated to decreased survival probability (HR 4.52).
To the best of our knowledge, this is the first evidence supporting ENT1 as a prognostic marker in resected, chemotherapy-naïve PAC patients. Based on our results, we speculate that ENT1 expression is lost during the tumor progression process and could be incorporated into the PAC patient risk standard evaluation.
Introduction
Pancreatic Adenocarcinoma (PAC) is one of the most frequent gastrointestinal malignancies and currently ranks as the fourth leading cause of cancer death in developed countries [1]. Its incidence ranges between 8–12 in 100,000, and almost 50,000 new cases are diagnosed in the United States every year [2]. Most patients are diagnosed at advanced stages. About 50% already have distant metastasis or display locally advanced disease. This entails a poor survival, close to 30% at one year, and only 6% at five years [3]. In recent decades, several clinical trials have aimed to improve clinical outcomes in PAC. Despite this, surgery remains the most effective treatment; unfortunately, this is accomplished in < 15% of cases [4]. The ESPAC-3 study demonstrated that Gemcitabine was the most effective adjuvant treatment for resected PAC, displaying similar survival compared to 5-Fluorouracil (5-FU) but less toxicity [5]. More recently, the ESPAC-4 trial showed that Gemcitabine/Capecitabine combination improves median survival up to 28 months versus Gemcitabine alone, supporting its use as the new standard of care [6].
The discovery of “companion diagnostics” and biomarkers with predictive (i.e., associated with treatment response) and prognostic (i.e., associated with independent-to-treatment survival) utility to guide treatment and surveillance is an area of active research. Nucleosides are glycosylamines formed by a nitrogenous base and a five-carbon sugar (either ribose or deoxyribose). These molecules play a variety of cell functions serving as precursors for nucleic acids (DNA and RNA), mediators of energy metabolism (in the form of ATP or GTP), and as ligands for purinergic receptors (like adenosine or inosine). Some of these compounds are hydrophilic and rely on their entry to the intracellular space to exert their biological functions. There are two classes of nucleoside transporters: Equilibrative Nucleoside Transporters (ENTs) and Concentrative Nucleoside Transporters (CNTs). Among ENTs [7], the best-characterized are human transporters ENT1 and ENT2. Studies have demonstrated that ENTs control the access of nucleosides and regulate the influx of therapeutics into cells. Indeed, ENT1 is the main transporter incorporating Gemcitabine into cancer cells. As explained above, Gemcitabine is the chemotherapy of choice for PAC; consequently, ENT1 expression has been postulated as a predictive biomarker for Gemcitabine response in patients [7–9]. Accordingly, a retrospective analysis demonstrated that high immunohistochemical ENT1 expression was associated with to better survival in patients treated with adjuvant Gemcitabine (17.1 months versus 26.2 months in low or high ENT1, respectively) [10]. Nevertheless, the same study indicated that ENT1 was not prognostic for the observational group. To date, no evidence supporting the prognostic utility of ENT1 in PAC has been reported.
Our group previously showed that ENT1 expression is a prognostic marker for pT2 gallbladder cancer patients [11]. Indeed, the median survival for low-ENT1 or high-ENT1 was 17.3 months or 28.7 months, respectively. To the best of our knowledge, these results are the first evidence supporting ENT1 as a prognostic biomarker in oncology.
Here, we expanded our studies and assessed the prognostic value of ENT1 expression in two independent cohorts of PAC patients with resectable disease, naïve to any adjuvant treatment following surgery. Our data suggest that ENT1 is a predictive marker for Gemcitabine response and a prognostic marker in PAC.
Materials and Methods
Patients and Samples: This study adhered to all relevant reporting recommendations for tumor marker prognostic studies (REMARK) [12]. Ethics Committees of all participating institutions (Pontificia Universidad Católicade Chile (PUC), Hospital Dr. Sótero del Río (HDSR), Hospital de Valdivia (HBV) and Fundación Arturo Lopez Perez (FALP)) approved an ethical request for waiving the Informed Consent, preserving the patients’ anonymity. Tumor samples were retrospectively obtained from patients with PAC who underwent surgery between 2003 and 2015 in one of the four aforementioned centers. We included patients with biopsy-confirmed diagnosis, age of 18 years or older, resectable disease, full clinical and pathological record, and tumor sample available for Tissue Microarray (TMA) study. Exclusion criteria were neo-adjuvant and/or adjuvant treatment and death within the first 30 days after surgery. An exploratory cohort of 87 patients was used to perform preliminary analyses. An independent cohort of 50 consecutively collected patients was used to validate the results.
Tumor samples were fixed in 4% neutral buffered formaldehyde and embedded in paraffin. The clinical-pathological features were obtained from records of each center and reviewed by a centralized pathologist at PUC. Clinical and pathological data were integrated in a central database, and each patient was randomly assigned with a code to maintain anonymity.
Tissue Microarray: Paraffin blocks for TMA were built using three representative cores from each patient sample. Sections of thickness 4 µm were cut and transferred to poly-L-lysine-coated glass slides. Blocks were deparaffinized with Histo-Clear (National Diagnostics, Atlanta, GA, USA), and rehydrated through an ethanol gradient. Heat-induced antigen retrieval was performed by immersing TMA slides in Tris-buffered saline containing EDTA (10 mM Tris, 1 mM EDTA, pH 9.0) for 20 mins at 99°C. Endogenous peroxidase activity was suppressed with phosphate-buffered saline containing 3% H2O2 for 20 min. Samples were incubated with a protein solution for 30 min to prevent non-specific staining and then incubated with a rabbit monoclonal antibody against human ENT1 (clone SP120; Spring Bioscience, Pleasanton, CA, USA; 1:100 dilution) for 45 min at room temperature. According to the manufacturer’s instructions, the primary antibody was detected with the VECTA-STAIN Elite ABC Reagent and Vector NovaRED (Vector Laboratories, Burlingame, CA, USA). Sections were rinsed and counterstained with hematoxylin, dehydrated through air exposure and Histo-Clear rinses, and cover-slipped.
Two independent, blinded pathologists with a large experience in pancreatic pathology assessed and quantified the immuno-reactivity for ENT1. The percentage of ENT1 positive cells and ENT1 staining were scored, and finally, the H-Score was calculated as previously described [13].
Statistical Analysis: A samples size of 73 patients was calculated for the exploratory cohort, considering the accuracy of 95%, power of 80%, the estimated ratio of 0.66 between samples with high ENT1 (H-ENT1) and low ENT1 (L-ENT), and an estimated relative hazard of 2.00 by following a previously described method [14]. Both the ratio between analysis groups and the relative hazard were estimated based on previous preliminary analysis. An adjusted power of 90% and a relative hazard of 3.00 was set after the exploratory analysis to calculate the validation cohort sample size in 36.
The best H-Score cut-off value to classify ENT1 expression as high (H-ENT1) or low (L-ENT1) was obtained from a simulated survival analysis performed on a randomly selected subgroup of 44 samples from the exploratory cohort by considering different H-Score thresholds. The simulation was 10-fold cross-validated on the remaining 43 samples from the exploratory cohort. Chosen H-Score was based on both the best survival discrimination and the least variability between the cross-validations. The Mann-Whitney test performed an analysis of differences in H-Score. Kaplan-Meier survival curves were plotted for patients grouped by their clinical-pathological characteristics and ENT1 expression. Overall survival was defined as the time from the date of surgery to death. The log-rank analysis was used to test the differences between survival curves.
Multivariate Cox Proportional Hazard analysis was used to estimate the effects of clinical-pathological variables, including gender, residual disease, differentiation, vascular invasion, neural permeations, pathologic T (pT), and N (pN) according to the AJCC classification. Finally, a backward selection was run to select the least number of variables needed to obtain a significant survival discrimination-function.
Statistical tests were two-tailed, and a p-value lower than 0.05 was considered statistically significant. Statistical analyses were performed in GraphPad Prism 6 (GraphPad, San Diego, CA, USA) and SPSS 20.0 (SPSS, Chicago, IL, USA).
Results
Cohort description: A total of four centers participated in this study and contributed with patients in similar proportions (PUC: 26%, HDSR: 28%, HBV: 24%, FALP: 22%). For the exploratory cohort, the median age at diagnosis was 64 years (IQ range 53–71). The median time of follow-up was 49 months. Only seven patients (8%) were alive at the end of the follow-up, with a median survival of 14 months (IQ range 7.3–29.4). Exploratory and validation cohorts did not display significant differences. Similarly, no differences in overall survival were found for sex, residual disease, tumor differentiation (well versus moderately/poorly differentiated), vascular invasion, neural permeations, pathologic T stage (pT1–T2 versus pT3–T4) or pathologic N stage is neither the exploratory nor the validation cohort.
Low ENT1 expression is associated with aggressive pathological characteristics: When analyzing patients grouped by individual clinical-pathological characteristics, ENT1 showed a differential expression for tumor differentiation (Figure 1A), vascular invasion (Figure 1B), and neural permeations (Figure 1C). ENT1 expression was higher in well-differentiated samples (H-score 46, 95% CI 1.68–90.32) versus moderately/poorly differentiated (H-score 12, 95% CI 4.47–19.19) (p-value 0.0239). Furthermore, samples with negative vascular invasion showed an H-Score of 28 (95% CI 10.62–44.38) versus 10 (95% CI 0.92–18.21) in those positive for vascular invasions (p-value 0.0002). Finally, ENT1 expression was higher in samples with negative neural permeations (H-score 36, 95% CI 4.45–66.80) compared to those with positive permeations (H-score 11, 95% CI 4.00–18.53) (p-value 0.0165).
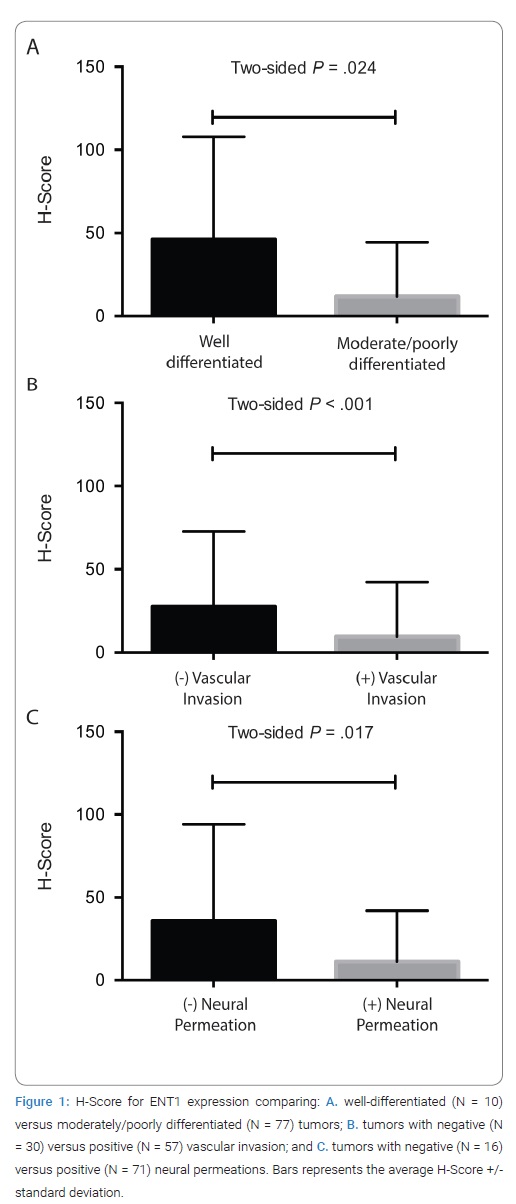
ENT1 expression modifies the pathological-dependent prognosis: Samples with an H-Score ≥ 10 were classified as H-ENT1 (32.2%), and those with H-Score < 10 were classified as L-ENT1 (67.8%), selecting the cut-off value as described in methods. The representative immunohistochemistry images of H-ENT1 and L-ENT1 tumors are shown in (Figure 1).
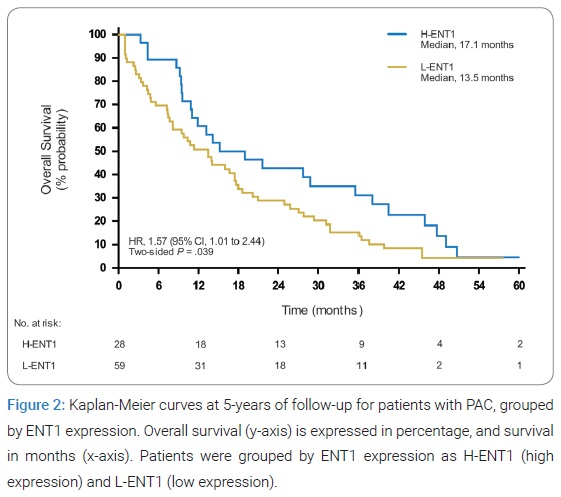
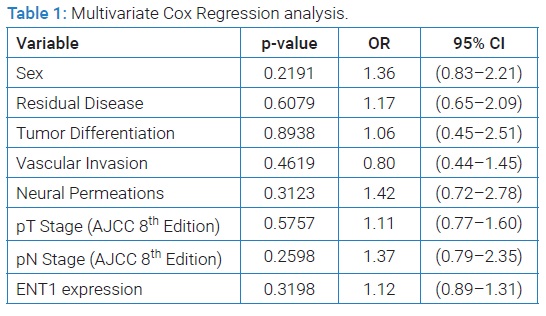
An analysis of the entire exploratory cohort (Figure 2) showed that patients with H-ENT1 or L-ENT1 had a median survival of 17.1 months or 13.5 months, respectively (HR 1.57, 95% CI 1.00-2.47, p-value 0.0385). Clinical-pathological characteristics and ENT1 expression were analyzed using a Multivariate Cox regression in order to weigh the independence of ENT1 in a survival model. Remarkably, none of the variables were individually significant (Table 1), suggesting that the prognostic value of ENT1 might depend on other clinical-pathological variables.
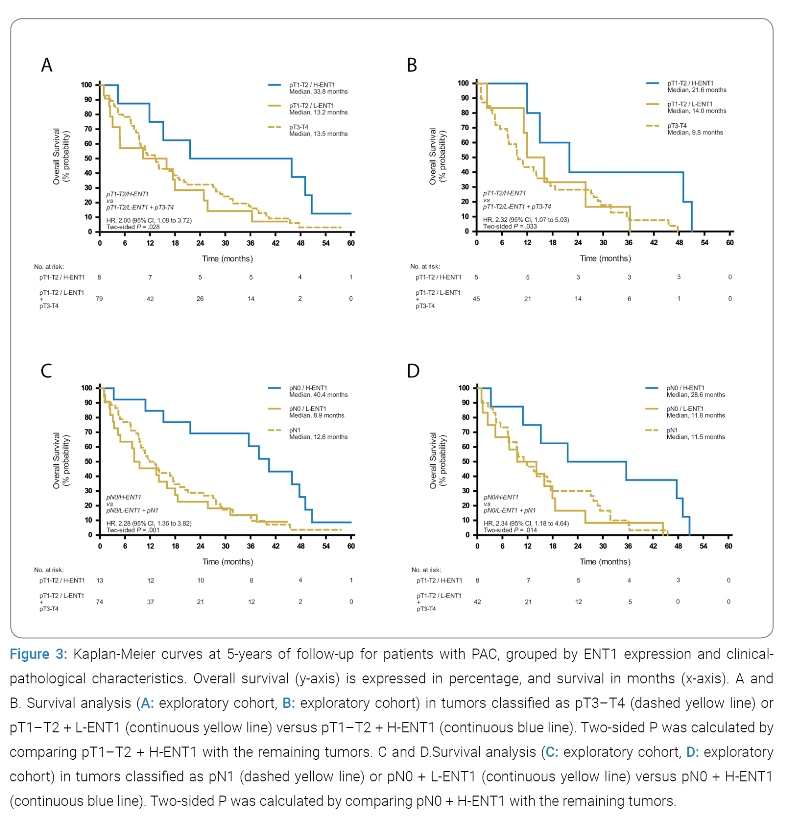
Consequently, a survival analysis, comparing H-ENT1 and L-ENT1, was performed in patients grouped by their clinical-pathological characteristics. Interestingly, when subgroups were analyzed by pT stage (Figure 3A), patients with pT1–T2 + L-ENT1 tumors had a similar survival (median survival 13.2 months) than those with pT3–T4 tumors (median survival 13.5 months), but together they had the worst prognosis than pT1–T2 + H-ENT1 tumors (median survival 33.8 months, HR 2.00, 95% CI 1.08–3.72, p-value 0.0284). Consistently, this result was similar in the validation cohort (Figure 3B), where pT1–T2 + H-ENT1 tumors had an improved survival (median survival 21.6 months) compared to pT1–T2 + L-ENT1 and pT3–T4 tumors (median survival 11.1 months, HR 2.32, 95% CI 1.07-5.03, p-value 0.0334).
When analyzing patients by the pN stage, the survival for pN0 + H-ENT1 patients (Figure 3C) was markedly superior (median survival 40.4 months) compared to those with pN0 + L-ENT1 and pN1 (median survival 11.7 months, HR 2.28, 95% CI 1.36–3.82, p-value 0.0014). As observed by pT stage analysis, this result was validated in the 50-patient independent cohort (Figure 3D), in which pN0 + H-ENT1 patients showed an improved survival (median survival 28.6 months) when compared to pN0 + L-ENT1 and pN1 patients (median survival 11.6 months, HR 2.34, 95% CI 1.18–4.64, p-value 0.0142). In summary, ENT1 expression impacts the risk of mortality in patients with pT1–T2 or pN0 tumors.
ENT1 expression determines survival in PAC: A backward selection by a Multivariate Cox Proportional Hazard analysis delivered ENT1 as the only significant variable to be associated with long-term survival. The survival probability obtained from the Cox Hazard model (including only ENT1 expression) was strongly correlated with real survival (R-Spearman 0.98) (Figure 4).
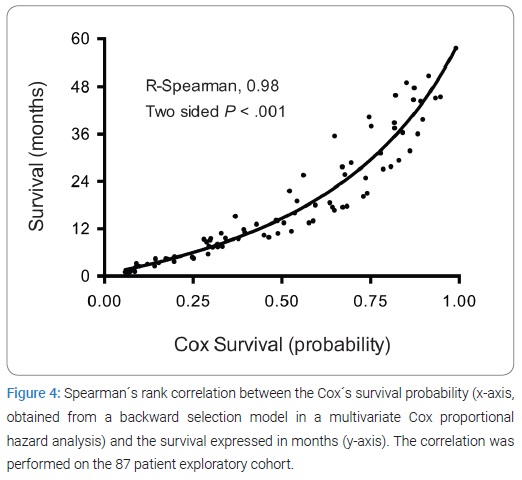
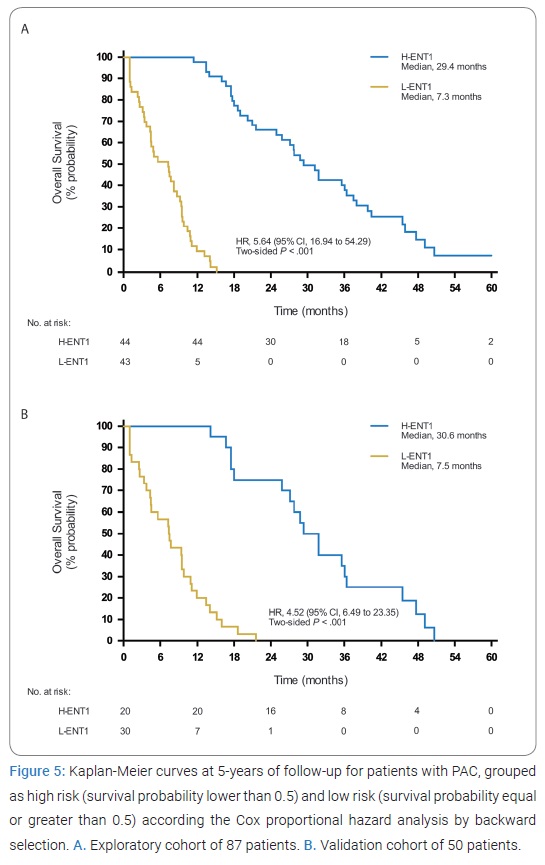
More importantly, high-risk patients (survival probability lower than 0.5) had a median survival of 7.3 months, while low-risk patients (survival probability equal or greater than 0.5) had a median survival of 29.4 months (HR 5.64, 95% CI 16.94-54.29, p-value < 0.0001) (Figure 5A), which was reproduced in the validation cohort (HR 4.52, 95% CI 6.49-23.35, p-value < 0.0001) (Figure 5B).
Discussion
Despite the significant advances for several malignancies in recent decades, long-term survival for PAC patients has remained at about 6%, making it one of the most aggressive neoplasms [2]. Understanding the biological basis of PAC development may bring novel therapeutic options for patients. Studies have previously demonstrated that ENT1 expression is a robust predictive biomarker for Gemcitabine-based chemotherapy response [7–9]. Herein, we report the first evidence supporting the role of ENT1 as a prognostic marker for chemotherapy-naïve resected PAC patients.
Our data show decreased ENT1 expression in PAC tumors with moderately/poorly differentiation, vascular invasion, and neural permeations suggesting ENT1 loss as the tumor increases its aggressiveness. We speculate that fast-growing tumors display larger necrotic areas as a result of hypoxia [15], which decreases ENT1 mRNA levels [16] as a compensatory mechanism. Hence, ENT1 transcriptional repression may increase extracellular levels of adenosine [17], a potent vasodilator that reduces tumor hypoxia [18]. As a secondary mechanism, the ENT1 promoter contains several putative binding sites for a number of transcription factors (which may act as repressors in malignancy), including SP-1 and an Estrogen Response Element (ERE) [19]. These transcription factors can respond to pro-oncogenic pathways, such as Src and MAPK. Particularly, SP-1 is a downstream effector of growth-factor receptors [20,21] and Src-related pathways [22]. Also, ERE can be regulated by membrane-associated tyrosine kinases receptors [23]. Finally, TGF-β1 is a known activator of the Epithelial to Mesenchymal Transition (EMT) that also inhibits ENT1 expression and activity [24]. Studies demonstrate that EMT is a characteristic feature of highly aggressive malignancies. Interestingly, a recent study on a modified PAC cell line (KPC) demonstrates that ENT1 expression is increased in Snail and Twist (genetic regulators of EMT) knock-outs [25].
Our results showed a strong association between lower ENT1 expression and poorer median survival rates in PAC patients. As explained, ENTs have the ability to regulate the transport of nucleosides, nucleobases, and therapeutics, an ability that has far reaching implications. For example, it is well documented that a decrease in nucleoside transporters [26] results in an increase in adenosine concentration [27] in cancer tissues. Adenosine has a wide range of biological effects in multiple organ systems determined by its interaction with adenosine receptors. Regarding tumor aggressiveness, adenosine regulates at least four key processes: First, activation of A1 receptors promotes cell proliferation by down-regulation of p27 (a CDK inhibitor) and up-regulation of CDK4 [28]. Second, adenosine can induce angiogenesis via an endothelium-dependent mechanism (expression of A2A receptors on endothelial cells is increased during early stages of lung cancer) [29] and an endothelium-independent mechanism (by increasing the macrophage-derived VEGF via A2A receptors) [30]. Studies demonstrate that blocking of adenosine receptor signaling reduces blood vessel density in the tumor parenchyma [31]. Third, adenosine seems to foster the immune evasion by increasing alternative macrophage-activation toward an M2-phenotype [32], as well as promoting the expansion of Myeloid-derived Suppressor Cells (MDSC) through A2B receptors [33]. Both M2-like cells and MDSC facilitate the transformation of pre-malignant cells and promote tumor growth and metastasis by suppressing immune surveillance [34]. Fourth, adenosine could activate EMT. In a cell-model of low-adenosine breast cancer, the cells showed a reduced adhesion to the extracellular matrix and low migratory proficiency, while a forced adenosine production conferred an increased invasive potential and adhesion [35,36]. Finally, signaling by A2B receptors reduces the development of cell-to-cell adhesions, increasing the potential for cell spread [37].
In summary, we speculate the association of low ENT1 expression with high-risk pathological factors and poorer prognosis could result from more aggressive disease. This could also serve as a driver for a cell transformation into a more invasive phenotype via an EMT. Evidently, more in vitro studies are required to elucidate the mechanisms supporting this hypothesis, including the modulation of ENT1 as a novel potential therapeutic target. To the best of our knowledge, this is the first evidence supporting the role of ENT1 as a prognostic marker in PAC patients with the resected, chemotherapy-naïve disease. Our results suggest that ENT1 expression could be incorporated into the standard risk evaluation of PAC patients. Ultimately, we expect that this work can help, in the future, to a better understanding of PAC pathophysiology, improving clinical outcomes on this devastating disease.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer Statistics. CA Cancer J Clin. 2007;57:43–66.
- American Cancer Society. Sources of Statistics 50 Screening Guidelines for the Early Detection of Cancer in Average-risk Asymptomatic People. 2015;52.
- Kamarajah SK, Burns WR, Frankel TL, Cho CS, Nathan H. Validation of the american joint commission on cancer (AJCC) 8th edition staging system for patients with pancreatic adenocarcinoma: a surveillance, epidemiology and end results (SEER) analysis. Ann Surg Oncol. 2017;24:2023–2030.
- Hand F, Conlon KC. Pancreatic cancer. Surg (United Kingdom). 2019;37:319–326.
- Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: A randomized controlled trial. JAMA. 2010;304(10):1073–1081.
- Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011–1024.
- Farrell JJ, Elsaleh H, Garcia M, Lai R, Ammar A, Regine WF, et al. Human equilibrative nucleoside transporter 1 levels predict response to gemcitabine in patients with pancreatic cancer. Gastroenterology. 2009;136(1):187–195.
- Morinaga S, Nakamura Y, Watanabe T, Mikayama H, Tamagawa H, Yamamoto N, et al. Immunohistochemical analysis of human equilibrative nucleoside transporter-1 (hENT1) predicts survival in resected pancreatic cancer patients treated with adjuvant gemcitabine monotherapy. Ann Surg Oncol. 2012;19 Suppl 3:S558–S564.
- Giovannetti E, Del Tacca M, Mey V, Funel N, Sara Nannizzi, Ricci S, et al. Transcription analysis of human equilibrative nucleoside transporter-1 predicts survival in pancreas cancer patients treated with gemcitabine. Cancer Res. 2006;66(7):3928–3935.
- Greenhalf W, Ghaneh P, Neoptolemos JP, Palmer DH, Cox TF, Lamb RF, et al. Pancreatic cancer hENT1 expression and survival from gemcitabine in patients from the ESPAC-3 trial. J Natl Cancer Inst. 2014;106(1):djt347.
- Espinoza JA, García P, Bizama C, Leal JL, Riquelme I, Weber H, et al. Low expression of equilibrative nucleoside transporter 1 is associated with poor prognosis in chemotherapy-naïve pT2 gallbladder adenocarcinoma patients. Histopathology. 2016;68(5):722–728.
- McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat. 2006;100:229–235.
- Pierceall WE, Wolfe M, Suschak J, Chang H, Chen Y, Sprott KM, et al. Strategies for H-score normalization of preanalytical technical variables with potential utility to immunohistochemical-based biomarker quantitation in therapeutic response diagnostics. Anal Cell Pathol (Amst). 2011;34(3):159–168.
- Machin D, Campbell MJ, Tan SB, Tan SH. Sample size tables for clinical studies, 3rd edition. Wiley-Blackwell. 2009.
- Mackey JR, Jennings LL, Clarke ML, Santos CL, Dabbagh L, Vsianska M, et al. Immunohistochemical variation of human equilibrative nucleoside transporter 1 protein in primary breast cancers. Clin Cancer Res. 2002;8(1):110–116.
- Eltzschig HK, Abdulla P, Hoffman E, Hamilton KE, Daniels D, Schönfeld C, et al. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med. 2005;202(11):1493–1505.
- Chaudary N, Naydenova Z, Shuralyova I, Coe IR. Hypoxia regulates the adenosine transporter, mENT1, in the murine cardiomyocyte cell line, HL-1. Cardiovasc Res. 2004;61(4):780–788.
- Sato A, Terata K, Miura H, Toyama K, Jr Loberiza FR, Hatoum OA, et al. Mechanism of vasodilation to adenosine in coronary arterioles from patients with heart disease. Am J Physiol Hear Circ Physiol. 2005;288(4):H1633–H1640.
- Abdulla P, Coe IR. Characterization and functional analysis of the promoter for the human equilibrative nucleoside transporter gene, hENT1. Nucleosides Nucleotides Nucleic Acids. 2007;26(1):99–110.
- Safe S, Abdelrahim M. Sp transcription factor family and its role in cancer. Eur J Cancer. 2005;41(16):2438–2448.
- Chen BK, Chang WC. Functional interaction between c-Jun and promoter factor Sp1 in epidermal growth factor-induced gene expression of human 12(S)-lipoxygenase. Proc Natl Acad Sci USA. 2000;97(19):10406–10411.
- Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23(48):7906–7909.
- Tokunaga E, Hisamatsu Y, Tanaka K, Yamashita N, Saeki H, Oki E, et al. Molecular mechanisms regulating the hormone sensitivity of breast cancer. Cancer Sci. 2014;105(11):1377–1383.
- Vega JL, Puebla C, Vásquez R, Farías M, Alarcón J, Pastor-Anglada M, et al. TGF-β1 inhibits expression and activity of hENT1 in a nitric oxide-dependent manner in human umbilical vein endothelium. Cardiovasc Res. 2009;82(3):458–467.
- Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527(7579):525–530.
- Pennycooke M, Chaudary N, Shuralyova I, Zhang Y, Coe IR. Differential expression of human nucleoside transporters in normal and tumor tissue. Biochem Biophys Res Commun. 2001;280(3):951–959.
- Gessi S, Merighi S, Sacchetto V, Simioni C, Borea PA. Adenosine receptors and cancer. Biochim Biophys Acta. 2011;1808(5):1400–1412.
- Mirza A, Basso A, Black S, Malkowski M, Kwee L, Pachter JA, et al. RNA interference targeting of A1 receptor-overexpressing breast carcinoma cells leads to diminished rates of cell proliferation and induction of apoptosis. Cancer Biol Ther. 2005;4(12):1355–1360.
- Ahmad A, Ahmad S, Glover L, Miller SM, Shannon JM, Guo X, et al. Adenosine A2A receptor is a unique angiogenic target of HIF-2α in pulmonary endothelial cells. Proc Natl Acad Sci USA. 2009;106(26):10684–10689.
- Ernens I, Léonard F, Vausort M, Rolland-Turner M, Devaux Y, Wagner DR. Adenosine up-regulates vascular endothelial growth factor in human macrophages. Biochem Biophys Res Commun. 2010;392(3):351–356.
- Barcz E, Sommer E, Janik P, Marianowski L, Skopinska-Rózewska E. Adenosine receptor antagonism causes inhibition of angiogenic activity of human ovarian cancer cells. Oncol Rep. 2000;7(6):1285–1291.
- Csóka B, Selmeczy Z, Koscsó B, Németh ZH, Pacher P, Murray PJ, et al. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J. 2012;26(1):376–386.
- Ryzhov S, Novitskiy SV, Biaggioni I, Dikov MM, Feoktistov I. Response to comment on “Adenosinergic Regulation of the Expansion and Immunosuppressive Activity of CD11b+Gr1+ Cells”. J Immunol. 2012;188(7):2930.
- Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182(8):4499–4506.
- Zhou P, Zhi X, Zhou T, Chen S, Li X, Wang Li, et al. Overexpression of Ecto-5’-nucleotidase (CD73) promotes T-47D human breast cancer cells invasion and adhesion to extracellular matrix. Cancer Biol Ther. 2007;6(3):426–431.
- Zhi X, Chen S, Zhou P, Shao Z, Wang Li, Ou Z, Yin L. RNA interference of ecto-5′-nucleotidase (CD73) inhibits human breast cancer cell growth and invasion. Clin Exp Metastasis. 2007;24(6):439–448.
- Ntantie E, Gonyo P, Lorimer EL, Hauser AD, Schuld N, McAllister D, et al. An adenosine-mediated signaling pathway suppresses prenylation of the GTPase Rap1B and promotes cell scattering. Sci Signal. 2013;6(277):ra39.
Keywords
Pancreatic adenocarcinoma; ENT; Gemcitabine; Prognostic
Cite this article
Nervi B, Salas SV, Roa JC, Jarufe N, Mondaca S, Garrido M, et al. Equilibrative nucleoside transporter 1 expression is a prognostic marker in pancreatic adenocarcinoma. Clin Oncol J. 2021;2(2):1–8.
Copyright
© 2021 Bruno Nervi. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).






