Abstract
COVID-19 caused by SARS-CoV-2 has spread across the globe, claiming millions of lives. However, many have survived after infection. There are multiple risk factors associated with the disease's fatality like age, immunity, gender, diabetes, organ transplantation, etc. Host immune response plays a critical role in the control of infection and pathogenesis during this viral infection. Therefore, any treatment that modulates host immune response should be critically analyzed before its implementation during this pandemic. Cancer patients receive systemic treatments to regress the tumor growth and have multiple side effects on host immune response. However, little is known about the impact of such anti-cancer treatments on vulnerability, severity, and mortality from COVID-19. This mini-review focuses on the current literature and highlights the probable increase in risks for cancer patients receiving systemic anti-cancer treatments during the COVID-19 pandemic.
Introduction
COVID-19 is caused by a novel enveloped RNA beta-coronavirus known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2, also called 2019-nCoV) [1]. Inhalation of novel coronavirus latches the viral particles through angiotensin-converting enzyme 2 (ACE2) receptor onto the host cells that infiltrate the upper portions of the respiratory tract damaging it [2]. In response, the host adaptive and the cell-mediated immune system play a critical role in clearing off the virus, reducing the severity of infection.
Infection with SARS-Cov-2 is assumed to function in tandem with a myriad of health challenges faced by treatment receiving cancer patients as host immune response is most critical in deciding the survival of COVID-19 patients. Most of the systemic cancer treatments trigger multiple molecular signaling pathways affecting the immune system. According to a study conducted on 414 randomly recruited patients with confirmed COVID-19 in Renmin Hospital of Wuhan University, abnormal cellular immunity and humoral immunity were key features of non-survivors with COVID-19. Neutrophilia, lymphocytopenia, low CD4+ T cells, and decreased C3 were immunity-related risk factors predicting mortality of patients with COVID-19 [3]. Hence, weak immunity increases the severity of the infection and the rate of mortality. To understand the manifestation of COVID-19 in cancer patients receiving immune-modulating systemic treatments, we must address the probable impact of these treatments on the pathogenesis of coronavirus disease.
Immune Response During SARS-Cov-2 Infection
COVID-19 is caused by the inhalation of fine droplets containing SARS-CoV-2. The efficient immune system senses the viral challenge to protect the host. To defend tissue against any inhaled pathogen, the airways are not only endowed with physical barriers such as a mucus layer over its entire surface but also a vast network of respiratory tract epithelial cells, dendritic cells (DC), and alveolar macrophages. These cells trigger pro-inflammatory downstream immune responses in the presence of viral particles recruiting more innate and adaptive immune cells to limit pathogen spread. Liao et al. [4]. Type of immune cells recruited and polarization of immune response decide the severity of any infection like depletion of tissue-resident alveolar macrophages in broncho-alveolar lavage cells has been reported to be associated with disease severity (45). Thus any cancer treatment that reduces their count can increase the severity of viral infection, making the patient more vulnerable to COVID-19. T cells play a pivotal role in generating an immune response against multiple cancers (Table 1) and controlling coronavirus infection. According to a recent study, conducted on COVID patients, non survivors had smaller lymphocyte count (0.69 × 109 /L vs. 1.20 × 109 /L), diminished T cells subsets [CD3+ T cells (277 vs. 814 cells/μl), CD4+ T cells (172 vs. 473 cells/μl), CD8+ T cells (84 vs. 262.5 cells/μl, P < 0.001), CD19+ T cells (88 vs. 141 cells/μl) and CD16+ 56 + T cells (79 vs. 128.5 cells/μl) (P < 0.001)] when compared to survivors of COVID-19 [3]. This study strongly indicates the protective role of lymphocytes, especially T cells, in controlling SARS-CoV-2 infection during COVID-19 pathogenesis [5,6]. Taken together, this indicates that immunosuppressive agents/treatments that suppress lymphocyte cell count, especially T cells, may be particularly detrimental in fighting COVID-19 and thus should be avoided in patients with cancer. This review will shed light on the probable effect of systemic anticancer treatments on a patient's vulnerability to developing coronavirus infection, rate of its severity, and mortality caused due to it.
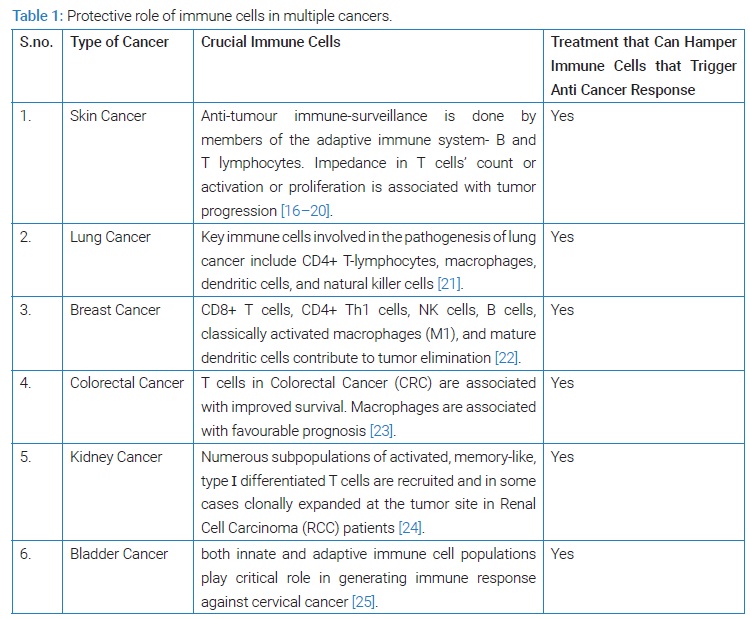
Anticancer Systemic Treatments
Radiotherapy: Radiotherapy is a systemic treatment given to cancer patients to regress tumor growth. However, radiation used in this therapy cannot differentiate between cancer cells and healthy cells. That is why it even hampers the number of immune cells like lymphocytes (T cells, B cells, and NK), which are among the most radiosensitive cells, followed by monocytes, macrophages, and Antigen-Presenting Cells (APCs), specifically Dendritic Cells (DC), which have comparatively higher radioresistance [7–9]. This treatment, therefore, leads to weakened host immunity. As lymphocytopenia act as a marker of high death risks of patients with COVID-19, such treatments should be avoided during a pandemic [10,11]. Lymphocytes, especially T cells, help fight against this viral infection as their number is higher in survivors than non-survivors of COVID-19 [3]. The protective role of T cells in SARS-CoV-2A can be well understood by a study that revealed that genes involved in T cell activation and function, such as MAP2K7 and SOS1, are down-regulated in the T cells of severe COVID-19 patients, and their expression of these genes returned to normal levels upon recovery [12]. Therefore, treatments like radiotherapy that lower patient's T cells, macrophages, or monocyte count make cancer patients more vulnerable to COVID-19 and decrease their chances of survival once infected.
Chemotherapy: Chemotherapy is another common treatment for cancer. Chemotherapy drugs are designed to target rapidly and uncontrollably dividing cancer cells, preventing them from growing further. Different combinations of medications are used depending upon the type of cancer as part of a chemotherapy treatment plan. Cancer patients receiving chemotherapy have significantly lower WBC leading to neutropenia, making it difficult to fight off viruses, bacteria, and other pathogens. This means the risk of infection is high. Patients with cancer are known to be at an increased risk for community-acquired respiratory viruses, such as influenza, due to their frequently observed immune-compromised state [13]. High fatality due to SARS-CoV-2 infection has been observed with cancer patients in Wuhan due to weakened immune response [14,15]. One reason for the increased risk of infection is a non-targeted systemic treatment that does not distinguish between cancer and normal cells, including immune cells.
Immunotherapy: One of the latest techniques used to treat cancer is immunotherapy, where the patients' immune system is boosted and trained to either slow or slop or destroy cancer cells. Immunotherapy is, therefore, very useful in the treatment of many types of cancer. Immunotherapy includes multiple approaches to treat cancer patients like T cell therapy, Monoclonal antibodies, and tumor-agnostic treatments, such as checkpoint inhibitors, etc. Unlike conventional methods like radiotherapy and chemotherapy, immunotherapy is targeted and improves immune response rather than impeding immunity.
Thus, immune-therapies initiate a self-sustaining attack against cancer cells by host immune cells that produce long-term clinical benefits or even a cure.
According to American Association for Cancer Research (AACR) 2020 Virtual Meeting: COVID-19 and Cancer, treatment with immune checkpoint inhibitors (ICIs) does not increase the risk of mortality in patients with COVID-19 and cancer. According to the article that was originally published on OncLive as, "Immunotherapy Use Does Not Correlate With Increased Mortality in Patients with COVID-19, Cancer." and was presented at 2020 AACR Virtual Meeting: COVID-19 and Cancer; July 20–July 23; the mortality rate of cancer patients with COVID-19 is almost same for individuals who received or did not receive immuno-oncology agents, i.e., nearly 8%. Immunotherapy in combination with conventional treatments could be a better option for serious cancer patients during COVID-19. Combination therapy will not hinder host immune response as much as chemo or radiotherapy when used alone. This will help cancer patients better fight against SARS-CoV-2A in case of infection and reduce the mortality rate in such patients.
Conclusion
Few studies from China have reported that cancer patients receiving systemic anticancer treatments have a higher risk of disease development than their counterparts who are not receiving anticancer treatment [26,27]. Wise choice of treatment for a cancer patient is of utmost importance because immunity plays a critical role in deciding the risk factor against SARS-CoV-2A infection. Systemic treatments that are non-targeted can increase the mortality rate in cancer patients, especially during COVID-19 pandemic. Targeted immunotherapy, either used alone or in combination, could be a better option because it boosts host immunity to stop or destroy cancer growth and reduces the chances of mortality due to SARS-CoV-2A infection.
Author Contributions
Dr. Shilpa Raghuvanshi Chauhan: writing-original draft, review and editing.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
Funding
The authors received no financial support for the research, authorship, or publication of this article.
Keywords
COVID-19, Cancer, Systemic treatments, Immune response, Risk factor
Cite this article
Chauhan SR. Systemic Treatments: is it making cancer patients more vulnerable to COVID-19? Clin Oncol J. 2021;2(2):1–4.
Copyright
© 2021 Shilpa Raghuvanshi Chauhan. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).

