Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) Risk and Associated Product Recall Approach: Description of a Patient-Centered Multidisciplinary Information, Decision Aid and Support Structure
* Rainville F;
Casault L;
Bergeron S;
Martel L;
Tremblay S;
Laferrière MC;
Dubé M;
Fournier MF;
Mimeault C;
-
* Rainville F: Breast Disease Center, CHU de Québec-Université Laval, Quebec City, Quebec, G1S 4L8, Canada.
-
Casault L: Department of Oncology, CHU de Québec-Université Laval, Quebec City, Quebec, G1S 4L8, Canada.
-
Bergeron S: Director for Professional Services, CHU de Québec-Université Laval, QuebecCity, Quebec, G1S 4L8, Canada.
-
Martel L: Oncology Nurse Navigator, CHU de Québec-Université Laval, Quebec City, Quebec, G1S 4L8, Canada.
-
Tremblay S: Director for Multidisciplinary Services, CHU de Québec-Université Laval, Quebec City, Quebec, G1S 4L8, Canada.
-
Laferrière MC: Quality Risk Management, CHU de Québec-Université Laval, Quebec City, Quebec, G1S 4L8, Canada.
-
Dubé M: Director for Specialized Ambulatory Services and Ophtalmology, CHU de Québec-Université Laval, Quebec City, Quebec, G1S 4L8, Canada.
-
Fournier MF: Director for Surgery and Perioperative Services, CHU de Québec-Université Laval, Quebec City, Quebec, G1S 4L8, Canada.
-
Mimeault C: Director for Nephrology and Oncology, CHU de Québec-Université Laval, Quebec City, Quebec, G1S 4L8, Canada.
-
Aug 10, 2021 |
-
Volume: 2 |
-
Issue: 1 |
-
Views: 2722 |
-
Downloads: 1971 |
Abstract
Introduction: Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) is a rare form of cancer mainly associated with textured breast implants and tissue expanders recalled in early 2019 in Canada. Currently, there are no consensus and no shared best practices to approach the BIA-ALCL associated social and psychological implications. This article aims to share the CHU de Québec-Université Laval’s practice in taking its social responsibility and approaching the BIA-ALCL associated recall as well as its psychological implications.
Materials and methods: The patient-centered multidisciplinary information, decision aid, and support structure that was put in place is described and comprises: a dedicated support phone line with staff members answering on business hours; an information and psycho-education group intervention specifically developed to inform, reassure and guide patients at risk of BIA-ALCL; a consultation with a member of the psycho-oncology team for decision aid and emotional support if desired; and a consultation with a plastic surgeon if desired.
Results: Women at risk of BIA-ALCL’s needs were met by the support phone line, group interventions, and consultations with plastic surgeons. Patient experience questionnaire analysis related to the group intervention shows that it is judged worth it, acceptable and relevant in the BIA-ALCL context and shows lower levels of distress, anxiety, depression, and information needs after the intervention.
Discussion: Women at risk of developing BIA-ALCL have important information and emotional needs that need to be addressed with a patient-centered multidisciplinary tailored approach to help them cope and support them in making informed decisions.
Conclusion: The shared model of practice, a support structure enabling access to individual and group interventions, allows to meet the needs of women at risk of BIA-ALCL, by providing relevant information, helping them cope with associated negative emotions and support them through their decision-making process.
Introduction
Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) is an emerging disease entity, a rare form of cancer, uniquely iatrogenic in nature, mainly associated with textured breast implants and tissue expanders with a certain textured surface. First described by Keech and Creech [1], the possible association between ALCL and breast implants was later examined by the U.S. Food and Drug Administration (FDA) in 2011 and confirmed in the following years [2,3]. In 2016, while the BIA-ALCL was recognized and classified as a novel lymphoma by the World Health Organization (WHO), the National Comprehensive Cancer Network (NCCN) established consensus guidelines for the diagnosis and treatment of the disease, which were revised and updated in 2019 [4]. In 2017, Health Canada published a first safety review concerning breast implants which described low incidence BIA-ALCL [5,6]. Health Canada's safety review was updated in February, 2019, concluding an increased risk of BIA-ALCL for textured breast implants and tissue expanders [7,8]. This led to the recall and suspension of Allergan's licenses for its Biocell textured breast implants and its Natrelle textured tissue expanders [9,10]. Currently, there is no consensus and no shared best practices to approach the BIA-ALCL associated social and psychological implications [11].
In early 2019, Quebec's Ministry of Health and Social Services (MSSS) asked all its establishments to review their records of patients who have had breast implant surgery since 1995 in order to identify those who have textured breast implants or textured tissue expanders. The MSSS requested to contact identified patients and provide information about the potential risk of BIA-ALCL and the symptoms or signs to monitor.
The purpose of this article is to share the CHU de Québec-Université Laval's practice in promptly taking its social responsibility, responding to the MSSS' request, and approaching the BIA-ALCL associated recall, as well as its psychological implications. More specifically, this article will describe the patient-centered multidisciplinary information, decision aid, and support structure that was put in place to help women who are at risk of developing BIA-ALCL, and present exploratory results about the structure's use and related to the achievement of the group intervention's objectives (inform, reassure and guide patients at risk of BIA-ALCL).
The CHU de Québec-Université Laval: The CHU de Québec-Université Laval is the largest University Hospital in the Canadian Quebec province and comprises five hospital centers, all situated in Quebec City. Recognized by Quebec's MSSS as a specialized and sub-specialized center, it provides health care, teaching, research, and evaluation. It also comprises the Deschênes-Fabia Breast Disease Center, which is dedicated to patients with breast problems and comprises a multidisciplinary and integrated oncology center where all aspects of breast cancer are treated, from prevention, genetics, and screening, to palliative care, through diagnosis, treatment, surgery, and breast reconstruction [12].
Design and implementation of a support structure: The CHU de Québec-Université Laval had to react in a timely manner to the MSSS' request, to Health Canada's recall, as well as to the media crisis that was going on in parallel. Indeed, a quantity of information from an array of sources that were not always reliable circulated in all forms of media, leading women to look for information, answers and support because some of them were experiencing high levels of insecurity and negative emotions such as anxiety and emotional distress [13]. The first actions that the organization took in early 2019 were to name a responsibility to coordinate the efforts to approach the BIA-ALCL issue. Moreover, mobilize its archives service staff to identify patients through official records and operating room binders in which stickers with the serial number of the products used were kept, who have had textured breast implants or tissue expanders surgically implanted in a CHU de Québec-Université Laval were operating room since 1995. In order to do that in a timely manner, the organization had to hire additional staff. Then, a tailored information letter (tailored by being personalized, and information given according to the type of implant and nature of the surgery undergone, namely cosmetic or breast reconstruction) was sent to each patient identified, giving them basic information on BIA-ALCL. Its potential risk and incidence, the symptoms or signs to monitor (breast swelling, pain, or a palpable mass), the procedure to follow in case of symptoms or signs (please consult your doctor), and giving them a phone line number to call if they had further questions, needed support concerning this issue or were considering having their implants removed even the slightest.
Additionally, the BIA-ALCL issue was established as a priority for the CHU de Québec-Université Laval directors' board management committee, which held 21 meetings concerning the BIA-ALCL issue and participated in seven meetings with Quebec's MSSS in 2019. Six of its 19 directors were closely involved in designing and implementing the patient-centered support structure that would not only answer the MSSS' base request. However, that would go further by helping involved patients cope with associated negative emotions (anxiety, emotional distress, worry, and fear) and support them through their decision-making process of keeping their textured breast implants or removing them [13].
Indeed, women at risk of developing BIA-ALCL who only have access to scarce, unreliable information from an array of sources may face dramatically increased negative emotions producing suboptimal decisions [11]. In order to help women cope with these emotions and to support them in making informed decisions, Marra and colleagues [11] recommend not only clearly providing relevant and reliable information and tailored to patient characteristics but also implementing decision aids or health communication systems. This is what the support structure, which will be described in more detail in the next section, was aiming for.
Materials and Methods
Description of the information, decision aid, and support structure: The structure implemented comprises four elements that complement each other, the two first elements being the core of the structure:
- A dedicated support phone line;
- An information and psycho-education group intervention specifically developed to inform, reassure and guide patients at risk of BIA-ALCL;
- A consultation with a member of the psycho-oncology team if desired; and
- A consultation with a plastic surgeon if desired.
It is a multidisciplinary structure involving doctors, surgeons, nurses, psychologists, social workers, and the administrative staff who manage the dedicated support phone line. Note that any of the four elements of the structure can be accessed as needed, not in any particular order.
Dedicated phone line: The dedicated support phone line was put in place so that it would be available as soon as the first tailored information letter was sent. An administrative staff member is answering during business hours, giving basic support and information, and redirecting the person calling to the right professional for the right need if required. The support phone line aims to respond to every patient and never leave anyone with questions unanswered or with concerns unaddressed without referring them to the right professional and informing them about the group intervention and its aims for which they will receive an invitation to participate. Indeed, the staff member also has the task of taking the personal information of every person calling in order to invite and give them access to the group intervention.
Group information and psycho-education intervention: A single meeting information and psycho-education group intervention was developed with the aim of informing, reassuring, and guiding women with textured breast implants who are at risk of developing BIA-ALCL. Indeed, women exposed to the risk of BIA-ALCL are in a context of uncertainty which may cause particular anxiety, emotional distress, worry, and fear [11,13]. In that particular context, they must face the decision of keeping the implants or having them removed. This decision is often based on their estimation of their own risk of developing BIA-ALCL, which can be biased or overstated by their negative emotions, but also by misleading information from mass media, newspapers, or personal contacts on the frequency of the BIA-ALCL, or by personal or familial experiences with cancer. For those who have had a reconstruction surgery after breast cancer, which was the case for the vast majority of women treated in the CHU de Québec-Université Laval, having to face the risk of a new tumor creates a situation that is even more critical, emotionally charged and affectively driven [11,14].
In order to make an informed decision, women thus need to take the emotion out of the equation and have access to tailored, specific, and clear information about BIA-ALCL to be able to estimate their own risk of developing BIA-ALCL fairly. Moreover, all risks and benefits of keeping the implants or having them surgically removed must be considered [11,15].
The group intervention was delivered by a multidisciplinary team (comprising medical, nursing, and psycho-oncology staff) to women at risk of developing BIA-ALCL and some loved ones. It was divided into three parts. Firstly, an oncology nurse navigator from the Breast Disease Center, in charge of the introduction, started the presentation by describing the normal and expected reactions of women with textured breast implants who are at risk of developing BIA-ALCL, namely having questions, looking for answers, wanting the implants removed, as well as experiencing anxiety, emotional distress, worry, and fear. Then, the nurse came back at why women chose to have implants in the first place and discussed normal breast pain associated with all kinds of implants and different strategies that can be used to optimize comfort and pain control.
Secondly, a doctor in charge of giving tailored, specific, and clear medical information about BIA-ALCL, described the anatomy of breasts with implants as well as the characteristics, advantages, and disadvantages of different types of implants, textured or not. Then, the doctor explained possible common breast implant complications (including capsular contracture and breast illness) as well as the rarer BIA-ALCL. Next, he presented the symptoms or signs to monitor in prevention (breast swelling, volume increase, and pain because of significant accumulation of fluid around the implant skin rash, ulcer, or a palpable mass), as well as eventual diagnosis and treatment of BIA-ALCL. Finally, the doctor finished presenting the most recent data about its frequency, prevalence, and incidence and discussing recommendations for women with textured implants with symptoms (rapidly consult a doctor) or without symptoms (surveillance).
Thirdly, the psychologist’s presentation mainly targeted the issue of fairly estimating the risk of developing BIA-ALCL and making an informed decision accordingly. In order to do that, the importance of getting relevant and reliable information about BIA-ALCL and its risk was discussed, followed by a discussion about how to interpret probability and how to interpret physical symptoms realistically. A section about reassurance followed it: learning to tolerate uncertainty or negative thoughts and discussing anxiety management strategies (breathing, relaxation, meditation and mindfulness exercises, social support, healthy lifestyle, physical activities, and leisure). The psychologist finished by discussing how to make an informed decision and presented an existing decision aid tool to help with the Ottawa Personal Decision Guide [16].
Finally, the doctor came back to conclude the group intervention by informing participants about the possibility of having a consultation with a member of the psycho-oncology team for decision aid and emotional support and a consultation with a plastic surgeon if desired.
It is important to note that a total of five live interactive intervention groups were conducted in June 2019 and July 2019, allowing participants to interact with the team members and get answers to their questions. Lastly, a special video captured group was held to make it available to those who could not attend in person or reached the support structure later than July, 2019. The video is available online, in a private section protected by a password–so that only those who went through the structure and needed access can access it.
Consultation with psycho-oncology: The CHU de Québec-Université Laval has dedicated psycho-oncology team staff members who are competent in accompanying patients through decision-making processes, offering emotional support, counseling, or brief psychotherapy [17]. These resources were made available for decision aid and emotional support at the request of any women treated at the CHU de Québec-Université Laval at risk of developing BIA-ALCL. In addition, should any women develop BIA-ALCL, these resources would be available to support them.
Consultation with plastic surgery: Many plastic surgeons conducted breast implant surgery at the CHU de Québec-Université Laval since 1995. Active plastic surgeons agreed to offer consultations for reviewing the patient’s comprehension about BIA-ALCL risk, discussing non-surgical and surgical options, and plan treatment in a shared decision patient-centered approach at the request of any women treated at the CHU de Québec-Université Laval at risk of developing BIA-ALCL. Active plastic surgeons were even accepted to be available for patients initially treated by a plastic surgeon that was no longer practicing.
Data: Data about the use of every element of the support structure (number of women) was gathered by the quality risk management team involved. In order to ensure the quality and identify areas of improvement for the group intervention, the quality risk management team gathered additional data with a patient experience questionnaire largely based on a pre-existing questionnaire developed to improve group interventions for breast cancer patients [13]. Participants were asked to complete the first questionnaire about their self-reported levels, on a single scale ranging from 1 to 10, of distress, anxiety, depression, and information needs before the start (pre) of the group intervention. Before leaving, participants were invited to complete another patient experience questionnaire asking again about their self-reported levels, on a single scale ranging from 1 to 10, of distress, anxiety, depression, and information needs but after (post) the group intervention, as well as asking about appreciation of the intervention and general commentaries in order to document and improve the group intervention [13].
Consent and ethics: Women consented and were completely free to use any element of the support structure. When consenting to participate in the group intervention, women were completely free to complete pre-post patient experience questionnaires and informed about its purpose of improving the group intervention. Because data was gathered around clinical interventions and continuous quality improvement in the health care process, ethical approval was not required or sought.
Analysis: Statistical methods: In order to describe the use of the support structure, descriptive data were used (number of women). In order to compare group intervention pre-post levels of self-reported distress, anxiety, depression and information needs, paired sample t-tests were performed (after checking normality using skewness (z = 0.88, p = 0.38) and kurtosis (z = –1.31, p = 0.19) tests). Concerning the appreciation of the group intervention, means (out of 10) were calculated.
Qualitative analysis of comments: Similar comments gathered with the patient experience questionnaire were grouped to identify areas of strengths and areas of possible improvements.
Results
Descriptive data: use of every element of the structure: Since the implementation of the information, decision aid, and support structure, data was compiled in order to monitor the use of every element (Figure 1).
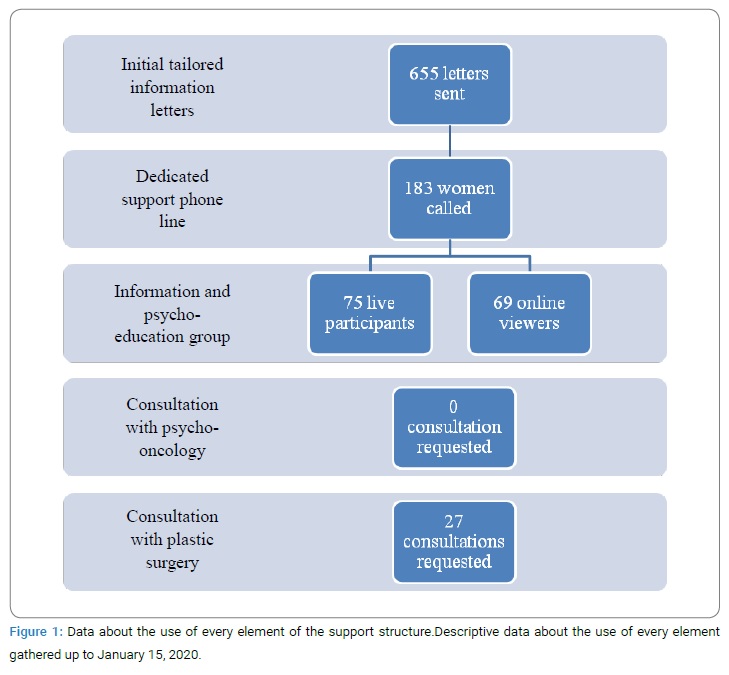
Note that at the time of the data collection, no woman treated at the CHU de Québec-Université Laval had been diagnosed with BIA-ALCL.
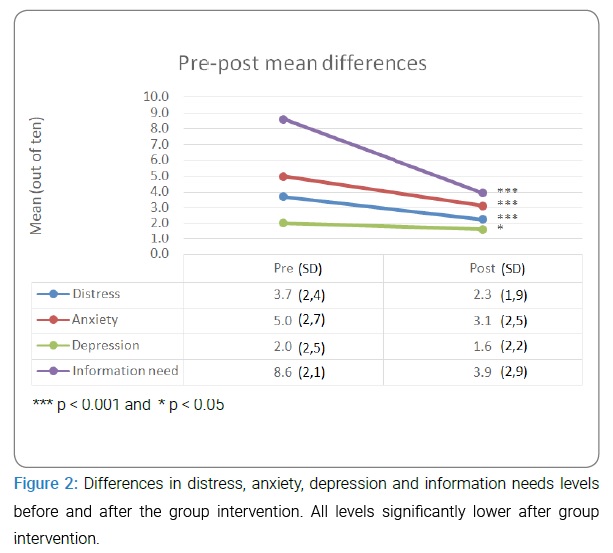
Quantitative results: information and psycho-education group: There were respectively 18, 14, 26, 14 and 3 participants in each live group, for a total of 75 participants, with a mean age of 56.8 years. Levels of distress, anxiety, depression and information needs were all lower after the group intervention, and all in a statistically significant way (distress (t(27) = 4.3, p < 0.001); anxiety (t(57) = 7.0, p < 0.001); depression (t(57) = 2.1, p = 0 .04); and information need (t(56) = 12.1, p < .001) (Figure 2)).
Appreciation of the group intervention was high for the group format (mean of 8.4 out of 10), for the relevance of the content (mean of 8.3 out of 10), and was very high for the feeling of participating in the group intervention being worth it (mean of 9.1 out of 10).
Qualitative results: Comments about information and psycho-education group: Out of the 33 comments left on the patient experience questionnaires, 10 were thanks, and six emphasized the reassurance provided, six emphasized the clarity, relevance, or usefulness of the information provided, two emphasized it is helpful to make an informed decision. Two whished that there were follow-up group sessions as new studies or recommendations arose, while seven stressed that they still had unanswered questions. Note that these seven participants were contacted by a team member to address remaining questions or concerns and to refer them to the right professional as needed.
Discussion
Women at risk of developing BIA-ALCL have important information and emotional needs that need to be addressed with a patient-centered multidisciplinary tailored approach in order to help them cope and support them in making informed decisions by clearly providing relevant and reliable information and tailored to patient characteristics, emotional support and decision aids [11]. The support structure that was put in place does that, and results show that women at risk of BIA-ALCL’s needs were met by the support phone line, group interventions, and consultations with plastic surgeons. In the CHU de Québec-Université Laval’s experience, no individual consultation with a psycho-oncology staff member was requested, highlighting that the emotional needs were met by the support phone line and the group intervention.
The group intervention’s objectives were to inform, reassure and guide patients at risk of BIA-ALCL. Data suggests that the group intervention was not only judged worth it, acceptable and relevant but that its objectives were met: it contributed to lower levels of distress, anxiety, depression, and information needs while guiding participants.
Concerning information, information need levels were the highest before the group and the one which diminished the most post-intervention. However, all information needs were not completely covered, which was also noted in the comments. These unmet information needs only seemed to be able to be met by a plastic surgeon, which is reflected by the 27 consultation requests with a plastic surgeon (14.8% of the 183 women in the care of the support structure). It can be noted that women did not need to participate in the group intervention or view it online to have access to a consultation with a plastic surgeon, but that the vast majority of the 27 requests involved women who had participated in live or viewed online group interventions. It is also important to note that most of the 27 women asked for a consultation with a plastic surgeon to decide to have their implants removed or not, rather than just having remaining informational needs.
Study limitations: The main limitations of this study are linked with a limited sample size and voluntary response bias. However, the study did not aim at producing findings that can be extrapolated but aimed at improving the support structure put in place and its group intervention through a continuous quality improvement in the health care process.
Conclusion
BIA-ALCL is an emerging disease entity, mainly associated with textured breast implants, for which the medical, social, and psychological implications must be taken very seriously. However, the authors noted no shared best practices to approach the BIA-ALCL associated social and psychological implications [11]. Therefore, this article shared a model of practice, a patient-centered multidisciplinary support structure that enables to meet the needs of women at risk of developing BIA-ALCL by providing accessible, clear, tailored, relevant, and reliable information, as well as by helping women at risk of BIA-ALCL cope with associated negative emotions and support them through their decision-making process of keeping their textured breast implants or having them removed.
It described a support structure, and an approach that can serve as a model used by quality risk management teams in case of other product recalls, putting people at risk of major health issues and implying a decision-making process. Because the structure and approach are patient-centered and have demonstrated a positive change while being efficient, notably because of its group intervention, our organization aims to submit it to Accreditation Canada as a Leading Practice to be exported to other health organizations.
Finally, the support structure and approach taken by the CHU de Québec-Université Laval were not only patient-centered and multidisciplinary–but they were also coherent with the CHU de Québec-Université Laval’s strategic directions, values, and priorities concerning patient experience: to provide our patients, partners in their care and services, and their loved ones, and experience marked with humanism, centered on their specific needs and respectful of their expectations [18].
References
- Keech JA Jr, Creech BJ. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg. 1997;100(2):554–555.
- U.S. Food and Drug Administration. FDA Update on the Safety of Silicone Gel-Filled Breast Implants. Silver Spring: U.S. Food and Drug Administration; 2011.
- U.S. Food and Drug Administration. Questions and Answers about Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL). Silver Spring: U.S. Food and Drug Administration; 2019.
- Clemens MW, Jacobsen ED, Horwitz SM. 2019 NCCN consensus guidelines on the diagnosis and treatment of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). Aesthet Surg J. 2019;39(Suppl_1):S3–S13.
- Health Canada. Breast Implants–Risk of Anaplastic Large Cell Lymphoma (BIA-ALCL). Ottawa: Government of Canada; 2017.
- Health Canada. Health Canada safety review finds low incidence of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) reported in Canada. Ottawa: Government of Canada; 2017.
- Health Canada. Health Canada will be updating its safety review of breast implants. Ottawa: Government of Canada; 2019.
- Health Canada. Summary Safety Review–Breast Implants–Assessing the potential risk of cancer (Breast implant associated anaplastic large cell lymphoma). Ottawa: Government of Canada; 2019.
- Health Canada. Health Canada suspends Allergan's licences for its Biocell breast implants after safety review concludes an increased risk of cancer. Ottawa: Government of Canada; 2019.
- Health Canada. Natrelle 133 tissue expander with magnafinder xact & 21g needle infusion set (2019-07-29). Ottawa: Government of Canada; 2019.
- Marra A, Viale G, Pileri SA, Pravettoni G, Viale G, De Lorenz F, et al. Breast implant-associated anaplastic large cell lymphoma: A comprehensive review. Cancer Treat Rev. 2020;84:101963.
- CHU de Québec-Université Laval. Qui sommes-nous? Québec: CHU de Québec-Université Laval; 2019.
- Martel L, Quirion J, Fillion L, Lessard A. Répondre aux besoins d’information de la clientèle : présentation d’une évaluation de groupe d’éducation (EGRE). Le Chuchoteur. 2020;8(2):1–3.
- Peters E, McCaul KD, Stefanek M, Nelson W. A heuristics approach to understanding cancer risk perception: Contributions from judgment and decision-making research. Ann Behav Med. 2006;31(1):45–52.
- Whitney SN, McGuire AL, McCullough LB. A typology of shared decision making, informed consent, and simple consent. Ann Intern Med. 2004;140(1):54–59.
- O’Connor AM, Stacey D, Jacobsen MJ. Ottawa Personal Decision Guides. Ottawa: Ottawa Hospital Research Institute & University of Ottawa; 2015.
- CHU de Québec-Université Laval. Oncologie psychosociale et spirituelle. Québec: CHU de Québec-Université Laval; 2019.
- CHU de Québec-Université Laval. Planification stratégique. Québec: CHU de Québec-Université Laval; 2019.
Keywords
Cancer; Breast implants; Patient-centered care; Product recalls; Psycho-education group intervention; Breast implant-associated anaplastic large cell lymphoma
Cite this article
Rainville F, Casault L, Bergeron S, Martel L, Tremblay S, Laferrière MC, et al. Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) risk and associated product recall approach: description of a patient-centered multidisciplinary information, decision aid and support structure. Clin Oncol J. 2021;2(1):1–7.
Copyright
© 2021 Rainville F. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).


