Use of a Commercially Available 12-Gene Assay in the Treatment of Early-Stage Colorectal Cancer: Results from a Nationally Representative Survey of U.S. Oncologists
* Naoko Ishibe Simonds;
de Moor JS;
Filipski KK;
Gray SW;
Schrag D;
Roberts MC;
Freedman AN;
-
* Naoko Ishibe Simonds: Consultant to the Scientific Consulting Group Inc., NJ, USA.
-
de Moor JS: Division of Cancer Control and Population Sciences, National Cancer Institute, MD, USA.
-
Filipski KK: Division of Cancer Control and Population Sciences, National Cancer Institute, MD, USA.
-
Gray SW: Department of Population Sciences, City of Hope, CA, USA.
-
Schrag D: Division of Population Sciences, Dana-Farber Cancer Institute, MA, USA.
-
Roberts MC: Division of Pharmaceutical Outcomes and Policy, UNC Eshelman School of Pharmacy, NC, USA.
-
Freedman AN: Division of Cancer Control and Population Sciences, National Cancer Institute, MD, USA.
-
Jan 15, 2021 |
-
Volume: 2 |
-
Issue: 1 |
-
Views: 2559 |
-
Downloads: 2059 |
Abstract
Background: The Oncotype DX Colon Cancer Assay is a 12-gene assay that measures recurrence risk in stage II and III colorectal cancer patients that help oncologists make treatment decisions. Although adjuvant chemotherapy for patients with resected stage III colorectal cancer is routinely recommended, its benefit remains controversial in stage II colorectal cancer patients; thus, the real-world use of Oncotype DX Colon is of great clinical interest. The purpose of this study was to investigate the use of Oncotype DX Colon by practicing U.S. oncologists in patients with early-stage colon cancer and factors associated with test adoption.
Methods: Using data from the 2017 National Survey of Precision Medicine in Cancer Treatment, weighted percentages were calculated to describe Gene Expression Profile (GEP) test use by physician demographics and practice characteristics.
Results: Of the 1,281 oncologists surveyed, 69.5% treated colorectal patients and, of those, 35.2% reported ordering Oncotype DX Colon. Oncologists who saw colorectal patients outside of an academic center and those treating 11 or more cancer patients per month were statistically significantly more likely to report using the Oncotype DX Colon test than those who did not have these characteristics.
Conclusion: In the U.S., a third of oncologists who treat colorectal cancer reported ordering Oncotype DX Colon. Future studies are needed to demonstrate whether GEP tests in patients with early-stage colorectal cancer have clinical utility. Additionally, because the benefit of adjuvant chemotherapy in this patient population is controversial, research on how these tests are being implemented in clinical practice is needed.
Introduction
Precision oncology is a rapidly evolving field that integrates information from genomic profiling to provide diagnostic, prognostic, and treatment strategies tailored to individual patients, maximizing treatment effectiveness and minimizing toxicity to improve health outcomes [1]. Moreover, oncologists tend to be early adopters of genomic tests, particularly when they predict clinical benefit [2,3]. In the context of other genomic tests, strong drivers of test adoption include evidence of clinical utility and availability of evidence-based clinical guidelines, whereas more limited drivers of test adoption include insurance coverage and cost-effectiveness [4]. However, except for Oncotype DX Breast, there are limited data on gene expression profile (GEP) test adoption and how provider or practice characteristics have encouraged or limited its use [5–7].
Colorectal cancer is one of the most common cancers [8] and, until recently, the risk of recurrence for stage II colorectal following resection was mainly based on traditional clinicopathological factors (e.g., T stage, number of nodes examined, tumor grade, etc.) [9–11]. Because these markers have not adequately characterized recurrence risk, GEP tests have been developed to better discriminate which patients would benefit from treatment. The Oncotype DX Colon Cancer Assay, which has been commercially available since 2010 [12,13], is a 12-gene assay that measures recurrence risk in stage II and III colorectal cancer patients to help oncologists make treatment decisions [14]. Although adjuvant chemotherapy for patients with resected stage III colorectal cancer is routinely recommended, its benefit remains controversial in stage II colorectal cancer patients; thus, the real-world use of Oncotype DX Colon is of great clinical interest. The purpose of this study was to investigate the use of Oncotype DX Colon assay by practicing U.S. oncologists and what factors may be associated with test adoption.
Methods
Data collection: We present data from the 2017 National Survey of Precision Medicine in Cancer Treatment, a nationally representative study of medical oncologists sponsored by the National Cancer Institute, National Human Genomic Research Institute, and the American Cancer Society. Of the 3,465 eligible oncologists with verified contact information, 1,281 responded for an overall cooperation rate of 38%. The survey captured information about oncologists’ use of various commercially available GEP tests as well as about their provider and practice characteristics. Details about the data collection and characteristics of the study population have been published elsewhere [3,15].
Oncologists were considered a user of Oncotype DX Colon if they reported ordering the test in the past 12 months. To put the results in context, the use of Oncotype DX Colon is compared with use of GEP tests for breast cancer (Oncotype DX Breast, Mammaprint, Prosigna, or Breast Cancer Index), which are supported by evidence of clinical utility. Oncologists who reported ordering any of the breast cancer GEP tests surveyed in the past 12 months were considered users.
Statistical analysis: Survey weights were calculated using the age, sex, and geographic location information available on the sample frame. The weights also adjust for the complex survey design by accounting for the probability of selection as well as the probability of non-contact and the probability of non-cooperation. The survey weights were applied in all analyses of the data to provide statistical adjustment so that respondents are representative of the U.S. population of practicing oncologists.
The weighted percentage of Oncotype DX Colon testing among oncologists was calculated overall and stratified by provider and practice characteristics. Provider characteristics included oncologists’ demographics, training in genomics, academic affiliation, and patient volume. Practice characteristics included practice setting and resources to support genomic testing.
Multivariable models were estimated to examine the independent association between each physician demographic and practice characteristic and the likelihood of Oncotype DX Colon testing, while adjusting for the other characteristics in the model. Results are presented as adjusted Odds Ratios (ORs) and adjusted weighted percentages (i.e., predicted probabilities) [13] with the corresponding 95% Confidence Intervals (CIs). All analyses were conducted using SUDAAN® release 11.0.1 (Research Triangle Park, NC).
Results
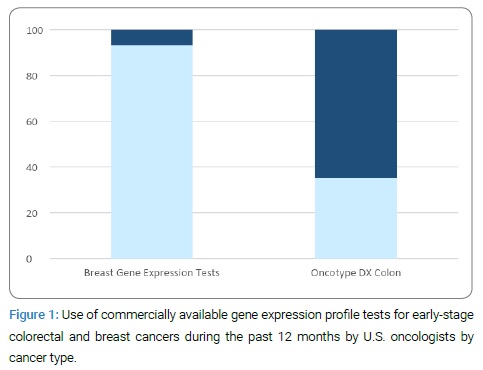
Use of Oncotype DX Colon test by provider and practice characteristics: Of the 1,281 oncologists surveyed, 69.5% (N = 890) reported treating colorectal patients. Overall, 35.2% of oncologists who treated colorectal cancer patients reported using Oncotype DX Colon tests to guide treatment. Oncologists who saw patients outside of an academic medical center (p < 0.001) or treated 11 or more colorectal patients per month (p < 0.001) were more likely to use Oncotype DX Colon tests compared with those who did not have these characteristics. Those who reported having a faculty appointment were more likely to use Oncotype DX Colon than those who did not hold a faculty appointment (p = 0.02). Oncotype DX Colon test use was also statistically significantly different by practice location (p = 0.03), where those who practiced in the Northeast or South reported ordering this test more than oncologists who practiced in the Midwest or West. No other characteristics were associated with test use (Table 1).
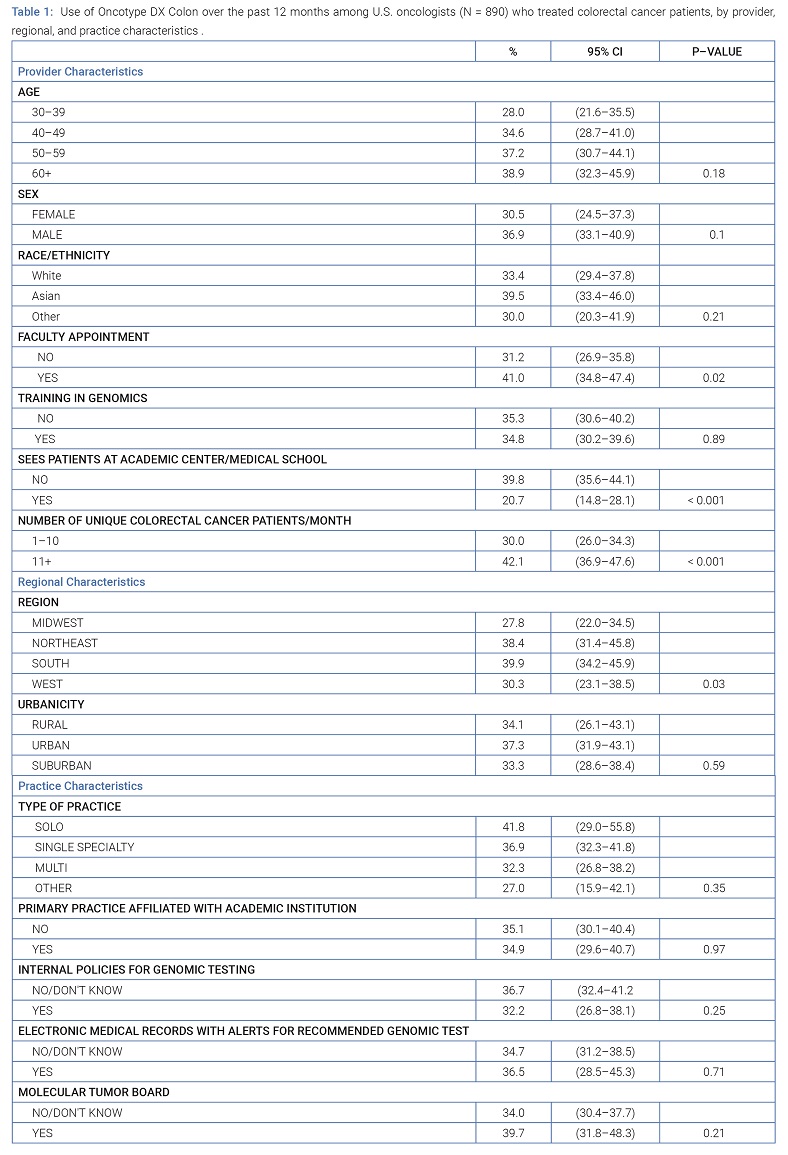
Frequency of gene expression test use by cancer type: Frequency of GEP test use varied by cancer type and is shown in Figure 1.
Of the U.S. oncologists surveyed, 71.7% (N = 919) reported treating breast cancer patients and 93.1% of these oncologists reported using GEP tests developed for breast cancer, which included Oncotype DX Breast, Mammaprint, Breast Cancer Index, and Prosigna. In contrast, 35.2% of oncologists who treated colorectal cancer patients used Oncotype DX Colon to guide treatment decisions.
Discussion
The use of GEP tests to guide treatment is increasingly important in oncology. For stage II colorectal cancer, clinicopathological factors have had limited ability to determine which patients would benefit from adjuvant chemotherapy, driving the development of assays that quantitatively estimate recurrence risk. In this study, we found that approximately a third of U.S. oncologists who treated colorectal cancer patients reported using Oncotype DX Colon when surveyed in 2017. A 2013 study of oncologists treating patients with stage II colorectal cancer found similar levels of use, suggesting that the use of Oncotype DX Colon has remained constant over time [16]. When compared to the use of GEP tests for breast cancer patients, the use of Oncotype DX Colon was more limited, likely reflecting the lower level of clinical utility evidence and more limited insurance coverage [17].
GEP tests to guide adjuvant treatment decisions in patients with early-stage breast cancer have been commercially available for over a decade, and multiple large trials have demonstrated both clinical validity and clinical utility [18–20]. The appropriate use of GEP tests for early-stage breast cancer is also clearly outlined in clinical guidelines [21,22], and these tests are covered by Medicare and all major U.S. commercial insurers [23,24]. In contrast, no studies have demonstrated clinical utility for Oncotype DX Colon. Despite a lack of evidence for clinical utility, the fact that over one-third of oncologists who treat colorectal cancer patients are using Oncotype DX Colon suggests that the adoption of new GEP tests is driven by a range of considerations, such as insurance coverage and patient preferences [12].
Our study found that provider and practice characteristics were also associated with Oncotype DX Colon test use for colorectal cancer. We observed greater use by oncologists who practiced outside of an academic center than oncologists whose practice was affiliated with an academic center. This observation may reflect the type of colorectal cancer patients seen in community practices in contrast to those treated at academic centers (i.e., early-stage disease versus more advanced disease), although we were not able to evaluate usage by stage of the disease. Also, not surprisingly, higher patient volume where oncologists who saw more than 11 patients per month were more likely to use these tests. This association is likely due to the greater probability of seeing at least one patient who would benefit from Oncotype DX Colon test results than in a practice seeing fewer patients. Additional research is needed to understand better these and other GEP test use drivers and the extent to which Oncotype DX Colon testing leads to better clinical outcomes.
This study had several limitations. We were only able to determine the percentage of providers who ordered the test at least once. We do not know how many tests were ordered, nor do we know what proportion of patients in whom the tests were used met the indication of use criteria. Another limitation is that we could not assess how oncologists used the test in specific clinical situations and whether they altered treatment recommendations based on test results. However, a prospective multicenter study reported changes in treatment recommendations based on recurrence scores [25]. Lastly, the cooperation rate was lower than that of previous surveys of physicians on the topic of genomic/genetic testing [26]; however, respondents were representative of the U.S. population of practicing oncologists in terms of age, sex, and geographic location, based on statistical adjustment for nonresponse using data available on the survey’s sample frame [27]. The large sample size also allowed us to analyze multiple factors associated with GEP test use.
Based on our survey, a third of U.S. oncologists reported ordering Oncotype DX Colon compared with over 90% of providers ordering breast GEP tests. Because the benefit of adjuvant chemotherapy in this patient population is controversial, research on how these tests are being implemented in clinical practice is needed. Additionally, although GEP test patterns in patients with early-stage breast cancer have been well documented [6,7,28,29], future studies are needed to demonstrate whether GEP tests in patients with early-stage colorectal have clinical utility.
Funding
Supported by the National Institutes of Health (contract No. HHSN261201400011I to Scientific Consulting Group for N.I.S.).
Conflict of Interest
Stacy W. Gray reported a consulting role with Grail. Deborah Schrag reported receiving personal fees from JAMA, speaking fees from Pfizer, and institutional support from Grail and Pfizer. The remaining authors have no conflicts to disclose.
Acknowledgments
The authors thank Timothy McNeel of Information Management Systems, Inc. for providing data analysis support.
References
- Schwartzberg L, Kim ES, Liu D, Schrag D. Precision Oncology: Who, How, What, When, and When Not? Am Soc Clin Oncol Educ Book. 2018;37:160–169.
- Schilsky RL. Drivers of change in cancer care. J Oncol Pract. 2016;10:315–316.
- Freedman AN, Klabunde CN, Wiant K, Enewold L, Gray SW, Filipski KK, et al. Use of next-generation sequencing tests to guide cancer treatment: Results from a nationally-representative survey U.S. oncologists. JCO Precision Oncology. 2018;1:1.
- Lai-Goldman M, Faruki H. Abacavir hypersensitivity: a model system for pharmacogenetic test adoption. Genet Med. 2008;10(12):874–878.
- Lynch JA, Berse B, Petkov V, Filipski K, Zhou Y, Khoury MJ, et al. Implementation of the 21-gene recurrence score test in the United States in 2011. Genet Med. 2016;18(10):982–990.
- Lynch JA, Berse B, Dotson WD, Khoury MJ, Coomer N, Kautter J. Utilization of genetic tests: analysis of gene-specific billing in Medicare claims data. Genet Med. 2017;19(8):890–899.
- Hull LE, Lynch JA, Berse BB, DuVall SL, Chun DS, Venne VL, et al. Clinical impact of 21-gene recurrence score test within the Veterans Health Administration: Utilization and receipt of guideline-concordant care. Clin Breast Cancer. 2018;18(2):135–143.
- GLOBOCAN 2018. Estimated cancer incidence, mortality, and prevalence worldwide in colorectal cancer fact sheet. International Agency for Research on Cancer, World Health Organization. (Accessed April 15, 2020) Available at: http://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact-sheet.pdf
- Vicuna B, Benson AB 3rd. Adjuvant therapy for stage II colon cancer: Prognostic and predictive markers. J Natl Compr Canc Netw. 2007;5(9):927–936.
- Petersen VC, Baxter KJ, Love SB, Shepherd NA. Identification of objective pathological prognostic determinants and models of prognosis in Dukes’ B colon cancer. Gut. 2002;51(1):65–69.
- Morris EJA, Maughan NJ, Forman D, Quirke P. Who to treat with adjuvant therapy in Dukes B/stage II colorectal cancer? The need for high quality pathology. Gut. 2007;56(10):1419–1425.
- Renfro LA, Zhang N, Lopatin M, Chao C, Alberts SR. Prospective evaluation of a 12-gene assay on patient treatment decisions and physician confidence in mismatch repair proficient stage IIA colon cancer. Clin Colorectal Cancer. 2017;16(1):23–30.
- Clark-Langone KM, Sangli C, Krishnakumar J, Watson D. Translating tumor biology into personalized treatment planning: analytical performance characteristics of the Oncotype DX Colon Cancer Assay. BMC Cancer. 2010;10:691.
- Webber EM, Lin JS, Evelyn PW. Oncotype DX tumor gene expression profiling in stage II colon cancer. Application: prognostic, risk prediction. PLoS Curr. 2010;2:RRN1177.
- Wiant K, Geisen E, Creel DV, Willis G, Freedman A, de Moor J, et al. Risks and rewards of using pre-paid vs. post-paid incentive checks on a survey of physicians. BMC Med Res Methodol. 2018;18:104.
- Parikh AR, Keating NL, Liu PH, Gray SW, Klabunde CN, Kahn KL, et al. Oncologists’ selection of genetic and molecular testing in the evolving landscape of stage II colorectal cancer. J Oncol Practice. 2016;12(3):e308–e319.
- Oncotype IQ, 2019. (Accessed May 2019). Available at: www.oncotypeiq.com/en-US/colon-cancer/healthcare-professionals/oncotype-dx-colon-recurrence-score/insurance-coverage-and-financial-assistance.
- Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Eng J Med. 2015;373(21):2005–2014.
- Cardoso F, van’t Veer LJ, Bogaerts J, Slaets L, Viale G, Delaloge S, et al. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;375(8):717–729.
- Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Eng J Med. 2018;379(2):111–121.
- Krop I, Ismaila N, Andre F, Bast RC, Barlow W, Collyar DE, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline focused update. J Clin Oncol. 2017;35(24):2838–2847.
- NCCN, 2019. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology, Breast Cancer Version 1.2019. (Accessed May 18, 2019). Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- Genomic Health, 2019. (Accessed May 18, 2019). Available at: https://www.genomichealth.com/en-US/oncotype_iq_products/oncotype_dx/oncotype_dx_breast_cancer.
- Agendia, 2018. (Accessed May 18, 2019). Available at: https://www.agendia.com/news/mammaprint-receives-positive-coverage-decisions-from-three-major-private-healthcare-insurers/
- Srivastava G, Renfro LA, Behrens RJ, Lopatin M, Chao C, Soori GS, et al. Prospective multicenter study of the impact of Oncotype DX Colon cancer assay results on treatment recommendations in stage II colon cancer patients. Oncologist. 2014;19(5):492–497.
- Wideroff L, Vadaparampil ST, Greene MH, Taplin S, Olson L, Freedman AN. Hereditary breast/ovarian and colorectal cancer genetics knowledge in a national sample of US physicians. J Med Genet. 2005;42(10):749–755.
- Klabunde C, Creel D, De Moor J, Freedman A, Geisen E, Wiant K, et al. Why don’t physicians respond to surveys? Insights from a non-response follow-back survey of oncologists. Annual Research Meeting of Academy Health. Seattle:WA; 2018.
- Losk K, Freedman RA, Lin NU, Golshan M, Pochebit SM, Lester SC, et al. Implementation of surgeon-initiated gene expression profile testing (Onco type DX) among patients with early-stage breast cancer to reduce delays in chemotherapy initiation. J Oncol Practice. 2017;13(9):e815–e820.
- Chang MC, Souter LH, Kamel-Reid S, Rutherford M, Bedard P, Trudeau M, et al. Clinical utility of multigene profiling assays in early-stage breast cancer. Curr Oncol. 2017;24(5):e403–e422.
Keywords
Oncotype DX Colon; Clinical utility; Insurance coverage; Adjuvant chemotherapy; Colorectal cancer
Cite this article
Simonds NI, de Moor JS, Filipski KK, Gray SW, Schrag D, Roberts MC, et al. Use of a commercially available12-gene assay in the treatment of early-stage colorectal cancer: results from a nationally representative survey of U.S. oncologists. Clin Oncol J. 2021;2(1):1–6.
Copyright
© 2021 Naoko Ishibe Simonds. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).


