Prevalence and Patterns of Gastrointestinal Cancers among Obese Patients, from a Teaching Hospital in Saudi Arabia
* Duaa J. Alhazmi;
Dina H. Alsohaibi;
Hamidh A. Almusayliem;
Ola A. Bukhari;
Aseel A. Alharbi;
Aisha A. Alharbi;
Saleh M. Aldaqal;
-
* Duaa J. Alhazmi: Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia.
-
Dina H. Alsohaibi: Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia.
-
Hamidh A. Almusayliem: Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia.
-
Ola A. Bukhari: Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia.
-
Aseel A. Alharbi: Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia.
-
Aisha A. Alharbi: Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia.
-
Saleh M. Aldaqal: Department of Surgery, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia.
-
Jun 10, 2020 |
-
Volume: 1 |
-
Issue: 2 |
-
Views: 3164 |
-
Downloads: 2219 |
Abstract
Objective: To determine the prevalence and patterns of gastrointestinal (GI) cancers among obese and non-obese patients.
Background: Obesity is associated with many comorbidities. Several studies concluded that there is an association between obesity and different types of cancers. But the prevalence and patterns of GI cancers among obese patients need to be investigated.
Methods: The current case-control study was performed using medical records of all adult patients who were diagnosed with GI cancers at King Abdulaziz University Hospital from January 2010 until May 2018, with a sample size of 834. We excluded participants who had a missing Body mass index (BMI) value. SPSS21 software package was used for data analysis.
Results: 532 patients with GI cancer included in the study divided into non-obese and obese based on their BMI. Obese patients represented 22.9% (n = 122) while 77.1% (n = 410) are non-obese. The mean age at diagnosis in obese was 56.63 years and in non-obese was 56.65 years. The mean BMI of obese was 34.18 kg/m2. There were more females in the obese group than non-obese (54.9% vs. 34.9%, P = 0.00007). The commonest tumor site in obese was colorectal cancer (CRC) followed by gastric and pancreatic cancers while in non-obese was CRC followed by gastric and esophageal cancers (P = 0.348). There was no difference in the death rate among obese and non-obese (27.9% and 34.1% , P = 0.194). Obese patients have higher positive lymph node than non-obese (P = 0.236).
Conclusions: Our study showed that GI cancers are common among obese females and the commonest sites are CRC,gastric and pancreatic cancers in obese than non-obese and screening obese female is highly recommended in our society.
Introduction
Obesity is defined as a body mass index (BMI) that is greater than or equal to 30 kg/m2 and it is considered worldwide to be a significant problem, with a drastically increased incidence in recent years [1]. Obesity has been associated with several illnesses such as cardiovascular disease, diabetes, and cancer [2]. The World Health Organization (WHO) categorizes BMI as underweight (<18.5 kg/m2), normal (18.5 kg/m2–24.99 kg/m2), overweight (25 kg/m2–29.9 kg/m2), and obesity ( ≥ 30 kg/m2) [3]. Additionally, the prevalence of obesity for both sexes was 13.2% worldwide, 19.5% in the middle east, and 35% in Saudi Arabia [4].
Gastrointestinal (GI) cancer affects the function of digestive system organs such as the stomach, colon-rectum, liver, esophagus, and pancreas [5]. However, the most common GI cancer is colorectal cancer (CRC) worldwide and in Saudi Arabia, while in developing countries, it is gastric cancer [6,7]. Several studies concluded that there is an association between high BMI and different types of cancer such as CRC [8], postmenopausal breast cancer, endometrial cancer, renal cell carcinoma, esophageal adenocarcinoma [9,10], pancreatic cancer and liver cancer [1], ovarian cancer, advanced prostate cancer, gall bladder cancer, and gastric cardia cancer [10,11]. However, a few studies showed that there is an inverse relationship between obesity and gastric non-cardia adenocarcinoma [10] and esophageal squamous cell carcinoma [11].
Pathologically, the majority of the CRC clinicopathological characteristics included adenocarcinoma type (52%), a tumor size > 5 cm, and lymph node metastasis [12]. Another study performed in adult males with rectal cancer showed that most obese patients had larger tumor size compared to non-obese people [13]. In contrast to distal gastric carcinoma (DG), proximal gastric cancer (PG) had a larger tumor size, a higher rate of lymph node metastases, and an advanced stage [14].
In 2006, a study reported that 4% of cancer deaths in men and 16%–20% of cancer deaths in women resulted from obesity [15]. Obesity and overweight led to an increase in postoperative mortality in the patient who underwent surgery for gastric cancer [16].
In Saudi Arabia, there is a lack of studies about the relationship between obesity and cancer. Thus, we aimed to determine the prevalence and patterns of GI cancer among obese and non-obese patients.
Material and Methods
The study was approved by the Institutional Review Board (IRB) of King Abdulaziz University (KAU). The present cross-sectional study was performed using the medical records of all adult cancer patients who presented to the Department of General Surgery of KAUH, Jeddah, the western region of Saudi Arabia, from January 2010 to May 2018. There were 834 cancer patients recruited into this study. The selected patients had GI cancer including esophageal, gastric, pancreatic, hepatic, and CRC and were over 18 years of age. Patients who had a missing BMI value were excluded.
The following data were extracted from each patient’s records as the demographic data: age, sex, nationality, comorbidities, living or deceased, history of other cancers, history of receiving chemotherapy and radiotherapy, anthropometric measurement (height, weight, and BMI), and pathological factors (tumor site, histopathological type, size, grade, lymph node status, tumor margin, and TNM stage). The patients were divided into groups based on their BMI.
The data were inputted into Microsoft Excel 2016 version15.25 (Microsoft Corporation by Impressa Systems, Santa Rosa, California, USA), and all statistical analyses were performed using Statistical Package for Social Science (SPSS) Version 21, (IBM Corporation, Armonk, NY, USA). Categorical variables, such as sex, BMI groups, and cancer site and frequency were estimated and analyzed using the chi-square test to examine the association between the different variables. For continuous variables, the mean ± standard deviation was determined, and the data were analyzed using a one-way ANOVA. The level of significance was set at p < 0.05.
Results
During the study period, 834 patients who were diagnosed with GI cancer at our hospital and 532 patients with GI cancer were included into the study. There were 302 patients who were excluded because of missing BMI values, the patient was diagnosed before 2010, or the patient was not a GI cancer patient.
The mean age, age at diagnosis, and BMI in the obese group were 59.41 (26–85) years, 56.63 (23–83) years, and 34.18 (30–48.4) kg/m2, respectively. The mean overall BMI was 26.33 kg/m2. (Table 1) summarizes the clinicopathological characteristics of participants according to the BMI. Among 532 patients, 410 (77.1%) had a BMI of < 29.99 kg/m2 and 122 (22.93%) had a BMI greater than 30 kg/m2.
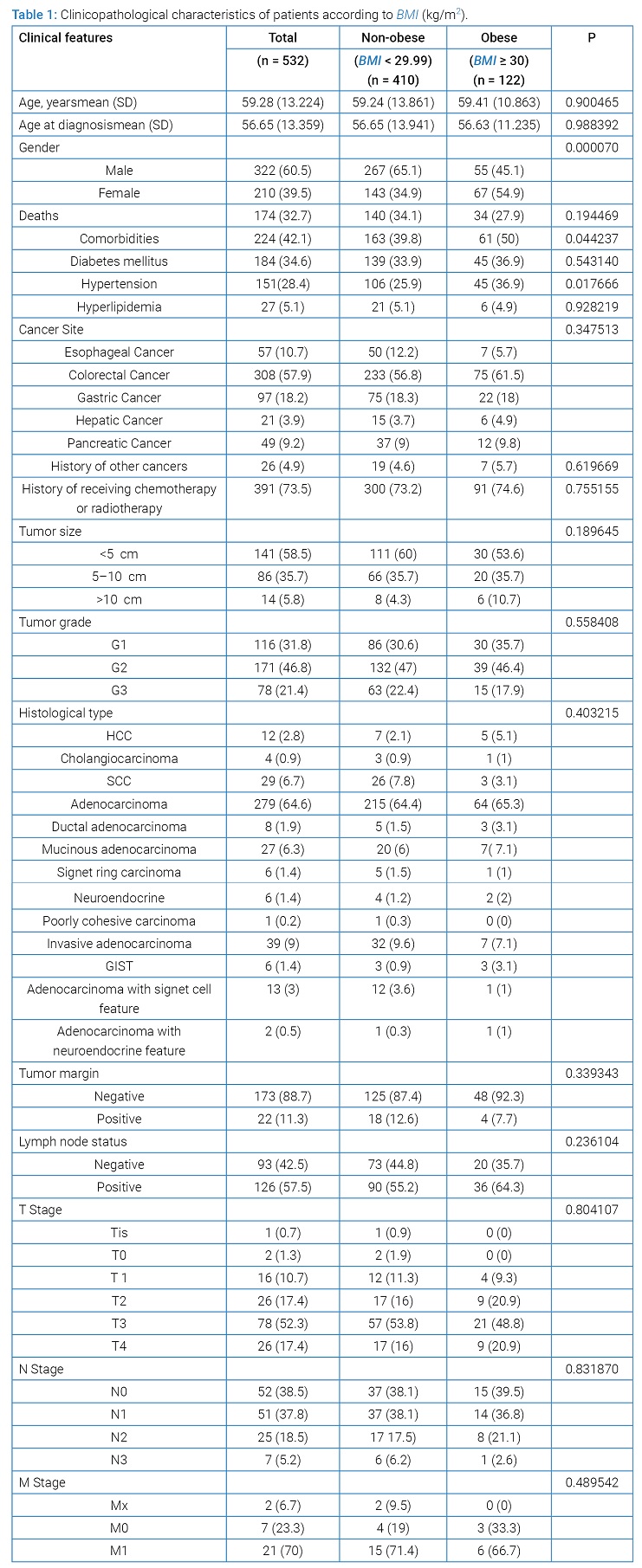
The only significant difference between obese and non-obese patients was their sex, where the greatest proportions of the obese patients were female (54.9% vs. 34.9%, P = 0.00007) and more obese patients had hypertension (36.9% vs. 25.9%, P = 0.017). There was no significant difference in the distribution of the patients’ BMI with age (P = 0.900), history of other cancers (P = 0.620), and history of receiving chemotherapy or radiotherapy (P = 0.755).
Diabetes (P = 0.543) was higher among obese patients compared to non-obese group. Additionally, the death rate among non-obese, and obese groups was 34.1%, and 27.9%, respectively (P = 0.194).
According to the cancer site, CRC had the highest prevalence (57.9%) followed by gastric cancer (18.2%). Similarly, CRC was the most common GI cancer type that affected obese patients followed by gastric cancer (P = 0.348).
Most obese patients had a tumor < 5 cm (P = 0.190) in size, with a moderately differentiated grade (P = 0.558), negative margin (P = 0.339), positive lymph nodes (P = 0.236) and relatively more advanced cancer (P = 0.804). About 42.9% of tumors that were larger than 10 cm were in obese patients (P = 0.190).
The most common histological type in obese patients is adenocarcinoma (P = 0.403). However, in non-obese patients, adenocarcinoma was the most common followed by invasive adenocarcinoma.
Discussion
In our current cross-sectional study, epidemiological and clinicopathologic characteristics and the prognostic factors for patients with different cancer locations were analyzed for obese patients with GI cancer. Participants in the present study had a 22.9% prevalence of obesity. Several studies reported that the prevalence of overall cancer was 33%, 7.7%, and 31.7% for obese people with a BMI > 30 in Sweden, Canada, and the United States, respectively [17–19]. In 2016, a study found a strong association between overweight/obesity and GI cancers including CRC and pancreatic cancer [20]. In almost all countries, education and other measures of socioeconomic status are inversely associated with the prevalence of obesity [21].
More obese females compared to obese males in our study had GI cancers. Similarly, two studies showed that, among obese persons, the rate of cancer was higher in females [17,22]. However, other studies reported that some types of GI cancers had a higher prevalence in men compared to women [23,24].
In this study, we found that in obese patients, the maximum age at diagnosis was 83 years, while the mean age in our study was 59.4 years and the mean age at diagnosis was 56.63 years. While some studies showed that the mean age of GI cancer diagnosis was 60.7–71 years for esophageal cancer, 66.8–73.1 years for gastric cancer [25], and > 50 years for CRC [26]. All these results suggest that the elderly are at higher risk of comorbidities and cancers.
Previous studies concluded that the obese population is more likely to develop CRC [27] and esophageal cancer [21]. Moreover, we found that CRC, gastric cancer, and pancreatic cancer were the three most frequent types of cancer in obese patients. However, in non-obese people, CRC was the most common followed by gastric and esophageal cancers.
We found no significance between histopathological features and BMI, but we observed that there was a relatively higher frequency of obese patients who had positive lymph node and negative tumor margins compared to non-obese. Additionally, 42.9% of tumor > 10 cm wasin obese patients. Overall, we found that most tumors were < 5 cm in all patients. Similar to our findings, one study conducted in gastric cancer patients showed that most tumors were < 5 cm [14].
Our study has several strengths. First, most of the results in the GI cancer field were similar to our findings with slight differences in the rates; this supports the good quality of our data, which can be used in another statistical study. Additionally, our results are based on clinical records, which supports conducting another study using the same sample. This is the first study done in Saudi Arabia about the prevalence of obesity among GI cancer patients.
However, there are several limitations to our study that should not be neglected. First, there were no data available on BMI and pathological reports, which could be overcome by changing the study design to a prospective study. In Saudi Arabia, the practice of autopsy is underestimated because of our religious and cultural beliefs, so most of our patients that had missing pathological reports were deceased before their tumor was resected or the biopsy was taken. Second, the sample size was inadequate because we investigated only five GI cancers at one hospital. We assume that the effect of obesity on patient outcomes might reach statistical significance with a larger sample size. Thus, we suggest conducting the study in several regions in Saudi Arabia and including all types of cancer.
Conclusion
The most common GI cancer site in obese patients are colorectal, gastric and pancreas in obese group. Moreover, Obese females were more than males;thus, we highly recommend screening of obese female in our society.
References
- Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol. 2012;56(3):704–713.
- Martin-Rodriguez E, Guillen-Grima F, Marti A, Brugos-Larumbe A. Comorbidity associated with obesity in a large population: The APNA study. Obes Res Clin Pract. 2015;9(5):435–447.
- Appropriate body-mass index for asian populations and its implications for policy and intervention strategies. The Lancet. 2004;363(9403):157–163.
- Abarca-Gómez L, Abdeen ZA, Hamid ZA, Abu-Rmeileh NM, Acosta-Cazares B, Acuin C, et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. The Lancet. 2017;390(10113):2627–2642.
- Sung JJ, Ng EK, Lin JT, Ho KY, Ji JF, Sugano K, et al. Digestive cancer management in Asia: position statements: a report on GI Oncology Summit in 2011. J Gastroenterol Hepatol. 2012;27(9):1417–1422.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.
- Bazarbashi S, Al Eid H, Minguet J. Cancer incidence in saudi arabia: 2012 data from the saudi cancer registry. Asian Pac J Cancer Prev. 2017;18(9):2437–2444.
- Al-Sharif MN, Fayi KA, Alobaidi AA, Alshamrani BA. Awareness of colorectal cancer among public in Asir region. J Family Med Prim Care. 2018;7(1):87–92.
- Ri M, Aikou S, Seto Y. Obesity as a surgical risk factor. Ann Gastroenterol Surg. 2018;2(1):13–21.
- Steffen A, Huerta JM, Weiderpass E, Bueno-de-Mesquita HB, May AM, Siersema PD, et al. General and abdominal obesity and risk of esophageal and gastric adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2015;137(3):646–657.
- Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. The Lancet. 2008;371(9612):569–578.
- Aldiab A, Al Khayal KA, Al Obaid OA, Alsheikh A, Alsaleh K, Shahid M, et al. Clinicopathological features and predictive factors for colorectal cancer outcome in the kingdom of saudi arabia. Oncology. 2017;92(2):75–86.
- Bokey L, Chapuis PH. Dent OF. Impact of obesity on complications after resection for rectal cancer. Colorectal Dis. 2014;16(11):896–906.
- Yu X, Hu F, Li C, Yao Q, Zhang H, Xue Y. Clinicopathologic characteristics and prognosis of proximal and distal gastric cancer. Onco Targets Ther. 2018;11:1037–1044.
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638.
- Struecker B, Biebl M, Dadras M, Chopra S, Denecke C, Spenke J, et al. The impact of obesity on outcomes following resection for gastric cancer. Dig Surg. 2017;34(2):133–141.
- Wolk A, Gridley G, Svensson M, Nyren O, McLaughlin JK, Fraumeni JF, et al. A prospective study of obesity and cancer risk (Sweden). Cancer Causes Control. 2001;12(1):13–21.
- Pan SY, Johnson KC, Ugnat AM, Wen SW, Mao Y. Canadian Cancer Registries Epidemiology Research G. Association of obesity and cancer risk in Canada. Am J Epidemiol. 2004;159(3):259–268.
- Greenlee H, Shi Z, Sardo Molmenti CL, Rundle A, Tsai WY. Trends in obesity prevalence in adults with a history of cancer: results from the us national health interview survey, 1997 to 2014. J Clin Oncol. 2016;34(26):3133–3140.
- Whiteman DC, Wilson LF. The fractions of cancer attributable to modifiable factors: A global review. Cancer Epidemiol. 2016;44:203–221.
- Cancer. IAfRo. Weight control and physical activity. Lyon: IARC press; 2002.
- Arnold M, Pandeya N, Byrnes G, Renehan PAG, Stevens GA, Ezzati PM, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16(1):36–46.
- Shin CM, Han K, Lee DH, Choi YJ, Kim N, Park YS, et al. Association among obesity, metabolic health, and the risk for colorectal cancer in the general population in korea using the national health insurance service-national sample cohort. Dis Colon Rectum. 2017;60(11):1192–1200.
- Koyanagi YN, Matsuo K, Ito H, Tamakoshi A, Sugawara Y, Hidaka A, et al. Body-mass index and pancreatic cancer incidence: a pooled analysis of nine population-based cohort studies with more than 340,000 japanese subjects. J Epidemiol. 2018;28(5):245–252.
- Anderson LA, Tavilla A, Brenner H, Luttmann S, Navarro C, Gavin AT, et al. Survival for oesophageal, stomach and small intestine cancers in Europe 1999–2007: Results from EUROCARE-5. Eur J Cancer. 2015;51(15):2144–2157.
- Ghalkhani F, Ghojavand M, Kashfi SMH, Jalaeikhoo H, Mojarad EN, Aghdaei HA. Clinico-pathological patterns of colorectal cancer patients in Tehran, Iran. Arvand Journal of Health & Medical Sciences. 2016;1(1).
- Ma Y, Yang Y, Wang F, Zhang P, Shi C, Zou Y, et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One. 2013;8(1):e53916.
Keywords
Obesity; Body mass index; Gastrointestinal cancer
Cite this article
Alhazmi DJ, Alsohaibi DH, Almusayliem HA, Bukhari OA, Alharbi AA, Aisha AA, et al. Prevalence and patterns of gastrointestinal cancers among obese patients, from a teaching hospital in Saudi Arabia. Clin Oncol J. 2020;1(2):1–6.
Copyright
© 2020 Duaa J. Alhazmi. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).

