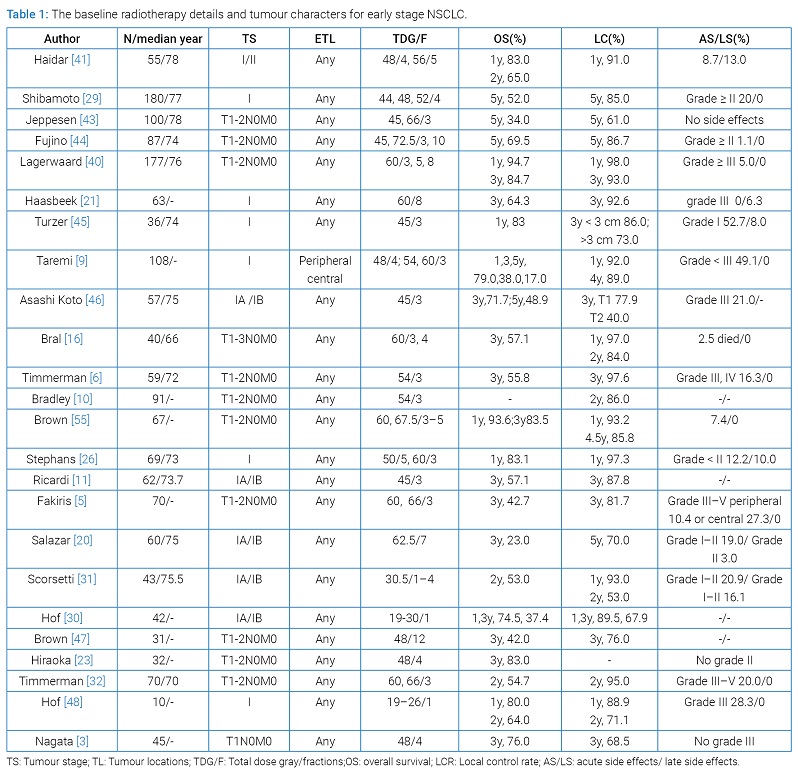
From these 24 studies, a total of 1654 early-stage NSCLC patients received SART. The radiotherapy details and tumour characters are shown in (Table 1).
Survival: The survival after SART treatment when considered for early-stage NSCLC of different studies is shown in (Table 1). In almost all studies, the median survival varied between 13.8 and 61.5 months, the 1,3 and 5-year overall survival rates ranged from 74.5%–94.7%, 23.0%–84.7% and 17.0%–69.5%, respectively. The 1,3 and 5-year disease-free survival were ranged from 70.2%–97.0%, 48.3%–81.0% and 23.0%–69.0%. When evaluating the factors effects the survival, the importance of tumour size was analyzed with respect to survival. The local progression-free survival for patients with T1 was longer than for those with T2 tumours (P = 0.006) [9,20]. The 1-year local progression-free survival estimate dropped below 80% for lesions with a diameter of more than 4 cm [21]. In patients with tumors ≤ 20 mm, overall survival was significantly higher than that in patients with tumors > 20 mm [23]. Simon, et al. [22] documented a significant difference in survival between patients with large (> 3 cm) and small (≤ 3 cm) tumours (P < 0.002). There was, however, no significant relationship between T-stage and overall survival in one study [22]. Therefore, it is hard to draw firm conclusions on the exact importance of tumour size for survival.
As for the location of tumour, previous studies found central (vs. peripheral) location did impact survival on both univariate and multivariate analyses [14,21,24]. The largest retrospective cohort found the 3-year overall survival for central and peripheral early-stage NSCLC was statistically not different (64% vs. 51%) [25], while another study found tumour location did not impact survival on univariate analysis for early-stage NSCLC [12].
Total radiation dose is another important prognostic factor for early stage NSCLC. Hiraoka M [26] reported that the overall survival of patients was significant positively correlated with the doses which they received the BED was less than 100 Gy, respectively. Overall survival at 3 years was 42% when the BED was less than 100 Gy, and 46% when it was over l00 Gy. In tumors, which received a BED of more than 100 Gy, overall survival at 3 years was 91% for operable patients, and 50% for inoperable patients [27]. Onishi, et al. [28] found improved overall survival rates with BED ≥ 100 Gy. They reported the most benefit in those with medically operable tumours, treated with BED ≥ 100 Gy. Patients with early stage NSCLC received radiation doses 10 Gy × 5 experienced a survival at 1 years in approximately 83.1% compared with 76.9% for patients receiving 20 Gy × 3 [29].
Local control: SART seems to be a safe and effective treatment for early stage NSCLC, which got high local control (Table 1). Among them, the 5-year local control rate (LCR), ranged from 83.0% to 86.7% [12,30]. The 3-year and 2-year LCR ranged from 40.0% to 97.6% and 53% to 95% [10,31–33]. With respect to local control, achieving a BED > 100 seems to be very important. The actuarial 2-years local tumor control was 85% for tumors treated with a BED > 100 Gy compared to 60% for tumors treated with a BED ≤ 100 Gy [34]. Onishi, et al. [28] found improved local control with BED ≥ 100 Gy. Stephans, et al. [25] reported that for the 10 Gy × 5 and 20 Gy × 3 cohorts at 1-year, local control was 97.3% vs. 100%. For patients with resectable early-stage NSCLC, 5-year actuarial local control rates were 84% for patients receiving a BED of 100 Gy or more and 37% for those receiving less than 100 Gy. Timmerman, et al. [10] reported a 3-year local control rate of 97.6%. Taken together the data indicated that better local control was obtained with the higher doses used in these studies. The local recurrence rate was 20% when the BED was less than 100 Gy and 6.5% when the BED was over 100 Gy. These data are support for better local control when total dose is increased [34].
Tumour size is one of the most important factors affecting both locoregional and distant control. In one of the studies it was seen that T2 lesions when compared with T1 lesions had significantly increased chances of local, regional and distant failures [35]. A similar study by Dunlap, et al. [36] concluded that SART for T2 NSCLC had a higher local recurrence rate. Hence, tumour size is an important predictor of response in SART. McGarry RC, et al. [37] found that excellent local control was achieved at higher dose cohorts. Patients with early stage NSCLC received radiation doses 10 Gy × 5 experienced a local control at 1 year in approximately 97.3% compared with 100% for patients receiving 20 Gy × 3 [29].
Patterns of failure: The patterns of failure after the treatment of SART include locoregional recurrence and distant metastases (Table 1). In early stage of NSCLC, distant metastases were the most common reason for treatment failure with SART [10,16,34,38]. In contrast, the frequency of locoregional recurrence was less, but varied considerably according to different reports [39,40]. The locoregional recurrence rate had ranged between 8.7%–37% and the distant metastasis rate of 8%–20% after a period of 1–2 years follow up [24,31,41,42]. On the basis of follow up after SART, median time to relapse varied from 21 to 30 months. The majority of recurrences occurred within 3 years after treatment. Thus, a 3-year follow-up is needed to estimate the recurrence rate after SART of early stage NSCLC. In our review of SART for early stage NSCLC studies, we found that the main pattern of failure was distant metastasis. This occurred in 9.7% to 29% of patients in studies with at least 30 months follow-up [41,43]. Nodal recurrences occurred in approximately 10% of patients [43]. Recurrences were associated with increased tumour size [44].
Side effects of radiotherapy: Published reports of SART for lung cancer describe a very low acute and late toxicity rate, with rates for grade 3 or higher toxicity being typically less than 4% [31–33,37,41,42,44–51]. In general, the common side-effects are mild to moderate (grade 1 to 2) and transient. The reported rate of grade ≥ 3 late toxicity was less than 10% in most studies. Most of the accumulated grade 5 events have occurred when patients received high-dose SART to centrally located tumours adjacent to meditational organs [9,52,53]. Timmerman, et al. [10] reported a rate of 12.7% grade 3 adverse events, 3.6% grade 4 adverse events, whereas Fakiris, et al. [9] reported that grade 3 to 5 toxicity occurred in 5 of 48 patients with peripheral lung tumors (10.4%) and in 6 of 22 patients (27.3%) with central tumors. Lagerwaard, et al. [41] found the toxicity was mild, with grade ≥ 3 radiation pneumonitis and rib fractures in 2% and 3%, respectively. Finally, there were other toxicities, such as oesophagitis, skin reactions, chest wall pain and general malaise such as fatigue [54].