Prognostic Significance of Aurora Kinase Expression and it’s Correlation with Other Apoptotic-like Proteins in Oral Tongue Squamous Cell Carcinoma
* Pablo Nenclares Pena;
Ana Ruiz Alonso;
Marina Alonso Riano;
Ballestin Caravilla C;
Perez-Regadera Gomez JF;
-
* Pablo Nenclares Pena: Department of Head and Neck Oncology, Royal Marsden NHS Trust Foundation, United Kingdom.
-
Ana Ruiz Alonso: Department of Radiation Oncology, University Hospital 12 de Octubre, Spain.
-
Marina Alonso Riano: Department of Pathology, University Hospital 12 de Octubre, Spain.
-
Ballestin Caravilla C: Department of Pathology, University Hospital 12 de Octubre, Spain.
-
Perez-Regadera Gomez JF: Department of Radiation Oncology, University Hospital 12 de Octubre, Spain.
-
Feb 28, 2020 |
-
Volume: 1 |
-
Issue: 1 |
-
Views: 3059 |
-
Downloads: 4135 |
Abstract
Background: Aurora kinases represent a family of serine/threonine kinases with an important role in cell division regulation. We evaluated the association of Aurora Kinase-A (AURKA) and Aurora Kinase-B (AURKB) with other apoptotic-like proteins, clinicopathological factors and survival in a cohort of Oral Tongue Squamous Cell Carcinoma (OTSCC) patients.
Methods: AURKA, AURKB, P53 and Survivin expression using immunohistochemistry, with a cut-off point of 10% was performed in 100 OTSCC patients.
Results: AURKA expression correlated with all clinicopathological factors and metastatic phenotype. AURKB upregulation was significantly associated with nodal status, depth of invasion ≥ 4 mm and distant metastasis. AURKA and P53 overexpression correlated with overall survival and disease-free survival. When adjusting by P53, AURKA was associated with shorter overall survival only in P53 over-expressors.
Conclusion: Increased expression of Aurora kinases in OTSCC patients is associated with poor prognostic factors and survival. The prognostic significance of AURKA protein expression is related to P53 status.
Introduction
Oral tongue squamous cell carcinoma is the most common malignancy of the oral cavity and the eighth most common neoplasm worldwide [1,2]. Its poor prognosis, even in early stages, and despite aggressive approaches, indicates the need for new prognostic tools that accurately predict the behavior of this tumor and which can aid in the selection of better treatment strategies [3–5]. A better understanding of the development and progression of OTSCC on the molecular level could lead to improved risk stratification of patients, facilitating more selective and individualized therapy. Since alterations of cell cycle control and mitosis may explain variations inaggression and prognosis in different tumor types, we sought to probe molecular markers involved in these pathways in OTSCC.
The aurora kinases represent a family of serine/threonine kinases that play a pivotal role in regulating cell cycle progression [6]. Aurora Kinase A (AURKA) regulates centrosome maturation, entry into mitosis, formation and function ofthe bipolar spindle, and cytokinesis [7]. Moreover, its phosphorylation supports the activity of other proteins involved in cell proliferation and survival, including important EGFR effectors such as AKT and RAS [8]. It has been described that AURKA activity is suppressed by P53 binding, leading to increased AURKA activation in P53–mutant tumors [9]. Aurora Kinase B (AURKB) contributes to the appropriate attachment and alignment of the mitotic spindle through interaction with a distinct set of partners [10]. AURKB has close interactions with survivin, a nuclear- and cytoplasmic-localised protein that inhibits apoptosis and regulates mitosis [11]. Additionally, AURKB phosphorylates P53, which accelerates the degradation of P53 by the proteasome [12].
Few studies have investigated aurora kinase expression in head and neck squamous cell carcinomas. Increased Aurora kinase concentration has been associated with more poorly differentiated phenotype, lymph node metastases, cellular proliferation and decreased patient survival [13–16]. In addition, a series of aurora kinase inhibitors have been produced for the past decades and have shown that inhibition of expression or activity of aurora kinases leads to suppression in cell proliferation, migration and invasion in cancer cells and inhibits the progress and growth of different tumor types [17–23]. Based on current investigations, MLN8237 (alisertib), one AURKA selective inhibitor, and the AURKB selective inhibitor AZD1152 are undergoing clinical trials for advanced solid tumors and hematologic malignancies [24–34]. However, clinical data for treatment of head and neck squamous cell carcinoma with aurora kinase inhibitors are scarce. For alisertib, investigators have reported disease stability in a few patients [35,36]. A follow-up phase 1 study of alisertib was done, followed by a planned phase 2 expansion study that included a cohort of patients with head and neck cancer (NCT01045421). A study is investigating the combination of cetuximab, alisertib and RT for patients with newly diagnosed head and neck cancers.
In the current study, we determined AURKA and AURKB expression by immunohistochemistry in 100 primary OTSCC. In addition, we examined whether there is an association of AURKA and AURKB overexpression with P53 and cytoplasmic survivin expression. Moreover, we correlated Aurora kinase expression with other known clinical and pathological prognostic factors and with patient survival.
Material and Methods
Patient selection and tissue samples: All tissue samples were obtained from diagnostic biopsies of patients who were diagnosed with primary OTSCC in the Department of Maxillofacial Surgery, Hospital Universitario 12 de Octubre, Madrid. In total, 100 primary, paraffin-embedded OTSCC were obtained from the archives of the Pathology Department. For all tumors, histopathologic and clinical follow-up data were available from follow-up examinations. OTSCC were classified according to the seventh edition of the American Journal Cancer Committee Staging System. Clinical data from the patients were retrieved from medical records. These data then were used to analyze the relation between molecular and clinical variables, such as smoking status (never smoker defined as less than 1 pack per year), drinkers (defined as 8 or more drinks for women, 15 or more for men), previous pre-malignant lesions, tumor and nodal classification, stage, histologic grade, Lymphovascular Invasion (LVI), Depth of Invasion (DOI), distant metastasis, Disease-Free Survival (DFS), and Overall Survival (OS). Generic informed consent at diagnosis was obtained from all individual participants included in the study.
Immunohistochemistry: Representative areas of neoplasia, excluding areas of necrosis, were selected and marked in paraffin blocks for each case. Subsequently, three Tissue Micro Arrays (TMA) were built extracting cylinders of the areas selected in paraffin blocks using the Manual Tissue Microarrayer MTA-1 (Beecher Instruments, Wisconsin, USA) with needles 1 mm thick. Every TMA contained 50, 40 and 34 cases, respectively. The data for the patients whose samples were on more than one TMA were consistent. Each case was duplicated within the same TMA, to minimize sampling errors or differences in staining. The TMA blocks were cut to 3 µm thickness. The sections were dried overnight to 37°C, dewaxed by immersing them in xylol and subsequently rehydrated by soaking them in decreasing graduation alcohol dilutions. All staining was done on a BOND III Autostainer (Leica Biosystems). Incubation with primary antibodies (1:100 AURKA polyclonal 1, Abcam; 1:50 AURKB clone ab3609, 1:100 P53 clon DO-7 Dako, 1:25 Survivinclon 12C4, Dako) was followed by secondary antibody and detection using bond polymer refiner detection. Cytoplasmic and/or nuclear immunoreactivity of AURKA, AURKB, P53 and Survivin was evaluated in each case. The TMA sections were reviewed independently by two expert pathologists who were blinded to the clinical data. Immunoreactivity was scored according to the percentage and intensity of cytoplasmic and/or nuclear staining of the positively stained tumor cells contained in each cylinder. The arithmetic mean of 2 cylinders immunoreactivity of each case was subsequently calculated to obtain a percentage of final stain. By using as cut-off the median expression level of each staining to distinguish the low-expressing (expression level lower than the cut-off value) from high-expressing patients (expression level equal or higher than the cut-off value) specimens with more than 10% of tumor cells stained were scored as positive.
Statistical analysis: Biomarker expression levels were compared between various groups in two class cases with t-test. Survival analysis was done for all patients. Disease-free survival was censored for patients who died without active disease. Overall survival was calculated from the date of biopsy until the date of death or last follow-up. The significance of AURKA and AURKB expression in predicting survival was determined by univariate and backward strategy multivariate Cox regression analysis (Stata 14). The Kaplan-Meier method was used to estimate survival and the log-rank method was used to compare the curves. The significance was set at 95% level. In light of the multiple testing in the univariate analysis P was adjusted using a Bonferroni correction (paltered ≤ 0.006).
Results
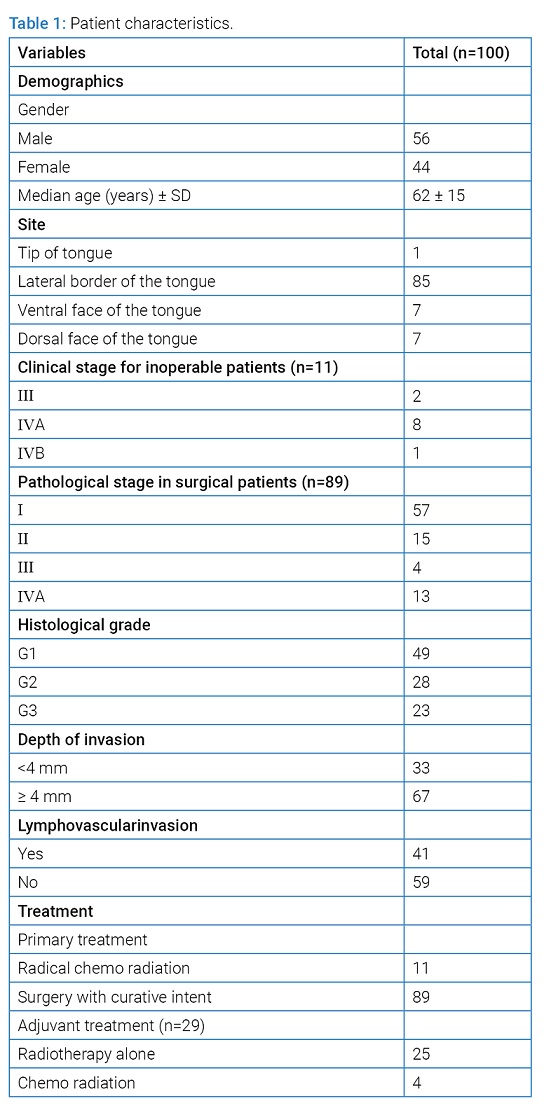
Patient and tumor characteristics: Of 100 patients included, the median age was 61.6 years (range 24 years to 89 years) and 56% were male. Overall, 67% of the patients were smokers or ex-smokers, and 34% were heavy drinkers. In 55 patients, the diagnosis of OTSCC was de novo, while 45 had pre-malignant lesions including leukoplakia, erythroplakia and lichen planus. A significant correlation was found between the smokingstatus and pre-malignant lesions (P<0.001). The most common location was the lateral border of the oral tongue (85%). The histological grade was predominantly grade 1(49%) and LVI was present in 41 subjects. Patients with previous pre-malignant lesions showed higher histological grades (P<0.001). DOI greater than or equal to 4 mm was found in 67 patients. Overall, 89 patients were initially treated with surgery, most of them with pathologic stage I (57%). Six of the 11 patients primarily treated with radical chemoradiation presented clinical stage IVA. The majority of patients were clinically no. There was a correlation between the histological grade, lymph node involvement (P=0.0172) and higher stages of the disease (P=0.0046). Positive nodal disease was associated with LVI (P<0.001) and DOI ≥ 4 mm (P<0.001). Detailed patient characteristics and histopathologic features are shown in (Table 1).
AURKA and AURKB expression and clinicopathologic characteristics: The immunohistochemical staining pattern for both AURKA and AURKB was predominantly cytoplasmic. AURKA showed additional staining in the cytoplasm in the majority of samples analyzed, which is consistent with previous immunohistochemistry studies [37]. Among the 100 patients, 80 and 82 tumor samples were acceptable for AURKA and AURKB analysis, respectively. Regrettably, the material was exhausted and therefore not technically acceptable to go back to the blocks more samples. Representative staining patterns by AURKA expression level are shown in (Figure 1).
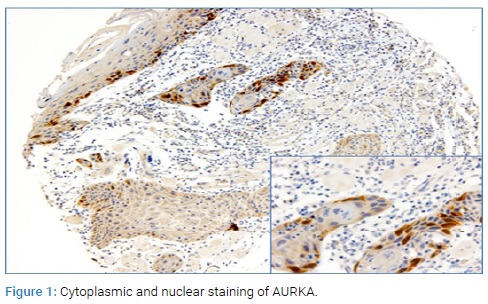
AURKA protein expression was significantly associated with the occurrence of regionally mph node (P=0.010), LVI (P=0.015), DOI ≥ 4 mm (P=0.032), tumor stage (P<0.001) and distant metastasis (P<0.001).
AURKB positive staining was significantly associated with nodal status (P=0.029), DOI ≥ 4 mm (P=0.031) and distant metastasis (P=0.013). A trend towards a correlation between tumor stage and AURKB expression was found (P=0.059).
Relationship between AURKA and AURKB expression and P53 and Survivin expression: Among the 100 patients, 95 and 93 tumor samples were acceptable for P53 and survivin analysis, respectively. A total of 22, 16, 50 and 54 subjects showed positivity for AURKA, AURKB, P53 and survivin immuno-staining, respectively. There was a correlation between AURKA and P53 expression (P=0.008). No correlation was found for AURKA and survivin expression (P=0.484). However, we found acorrelation between AURKB expression and Survivin positivity (P=0.02).
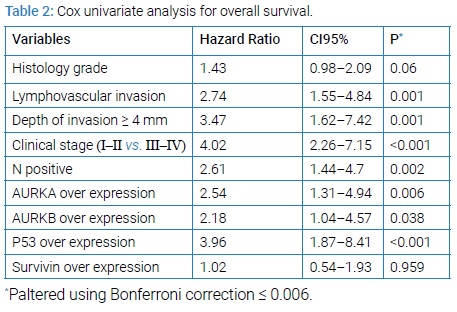
Association between survivaland AURKA and AURKB expression: With a median follow-up of 75 months, estimated 5 years DFS and OS rates were 58 and 60%, respectively. Except for tumor grading, all available clinicopathologic variables (LVI, DOI ≥ 4 mm, tumor stage and nodal status) were univariately significant for OS. AURKA and P53 overexpression were significantly associated with OS (Table 2).
When adjusting by P53 in the multivariate analysis, AURKA was associated with shorter OS only in P53-positive patients (HR 2.18, CI95% 1.07–4.45, P=0.031). Introducing this synergistic interaction in the model, multivariate OS analysis shows only a statistically significant correlation with tumor stage (HR 2.81, CI95% 1.4–5.68, p=0.004) and AURKA upregulation in P53-expressors (HR 3.34, CI95% 1.63–6.82, p=0.001). There was no correlation between AURKB, Survivin and OS. In (Figure 2).
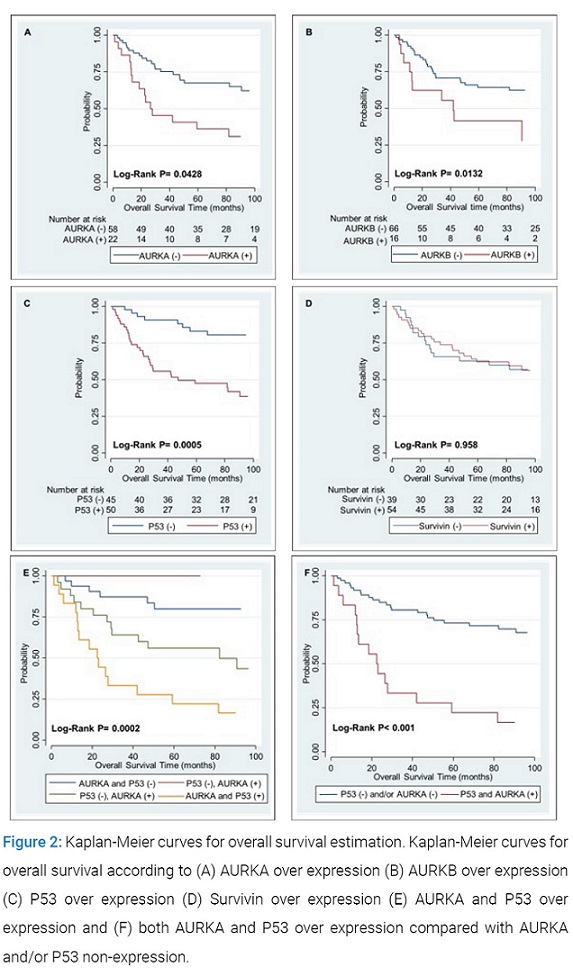
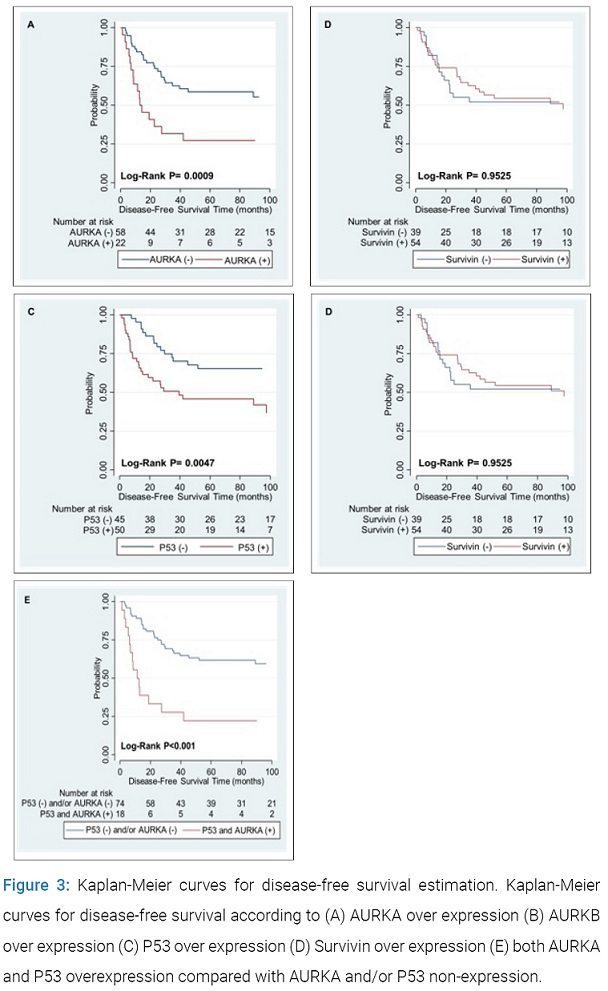
Six Kaplan-Meier survival curves for AURKA, AURKB, P53, Survivin and the combination of AURKA and P53 are plotted to show the results.
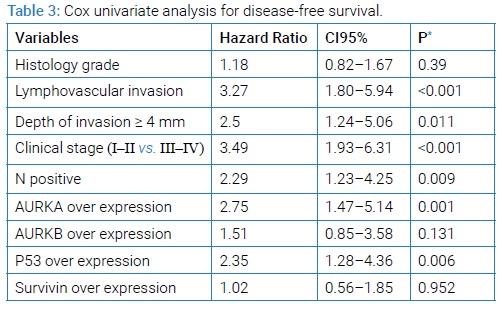
With regards to DFS, only LVI and tumor stage were univariately associated with outcome. Both AURKA and P53 overexpression were statistically associated with DFS (Table 3).
No significant correlation was found between AURKB and Survivin overexpression and DFS. When conducting the backward multivariate analysis, an interaction between the effect of AURKA and P53 was found. Taking this into account, only LVI and AURKA upregulation in P53-expressor were associated with DFS in the final multivariate model. Kaplan-Meier survival curves for DFS are shown in (Figure 3).
Discussion
The results of this investigation showed that overexpression of aurora kinases in OTSCC contributes to a poor prognosis. Both AURKB and AURKA overexpression is strongly correlated with worse clinicopathological features, a metastatic phenotype. In addition, AURKA upregulation is associated with shorter survival. Moreover, we found that AURKA overexpression is correlated with P53 and that upregulation in P53 presents a synergistic interaction with AURKA on its negative impact on OS and DFS. AURKB overexpression is associated to survivin upregulation and shows a trend towards a correlation with worse OS, however no statistically significant when adjusting by bonferroni correction.
Our findings suggest that AURKA upregulation is a common abnormality in OTSCC and may play an important role in its progression. Previous studies have described a correlation between AURKA overexpression and tumor progression and clinical aggressiveness in different tumorhistologie which was also found in our series of OTSCC patients [13,16,38–45]. Overexpression of AURKA leads to tetraploid cells with increased centrosome number that continue to divide in the absence of a functioning G1 checkpoint and P53 pathway [46]. The aneuploidy due to AURKA is detrimental and triggers mitotic checkpoints and apoptosis in normal cells [47]. However, cells with impairment in the P53 pathway are able to survive the tetraploid state andtend to have centrosome amplification, which can contribute to chromosome instability [48]. Moreover, AURKA upregulation contributes to cancer through the phosphorylation of P53 and its degradation by MDM2-dependent mechanisms [49]. By contrast, AURKA activity is suppressed by P53 binding, leading to increased AURKA activation in P53 tumors [50]. This is shown in our OTSCC series, in which AURKA overexpression is correlated with P53 overexpression. Moreover, these results further support our conclusion that AURKA overexpression is associated with worse survival dependent on P53 upregulation. Conversely, Mehra et al. [6] detected no significant association of alterations in P53 and the Aurora kinases when analyzing profiles in The Cancer Genome Atlas dataset. This could suggest that the relation between AURKA and P53 overexpression is only present in some specific locations with high prevalence of P53 mutation, such as the OTSCC. Alternatively, because of the association that these two proteins have with mitosis, this co-occurrence might be attributable to an underlying higher mitotic index in this subgroup of tumors.
AURKA overexpression and a metastatic phenotype were identified as being significantly associated in our study, as has been reported in previous studies [13,45]. Specifically, in head and neck cancers, Reiter et al. [16] showed AURKA mRNA upregulation was strongly correlated with distant metastases. Tong et al. [51] found that disruption of endogenous AURKA by gen-knockdown small interfering RNA technique in esophageal squamous cancer cell lines suppressed cell migration ability, which is an essential prerequisite for the metastatic process. These results support our conclusion that AURKA upregulation may contribute to tumor progression and clinical aggressiveness in OTSCC. In addition, subjects with both AURKA and P53 upregulation may represent the cohort of patient that benefit from the treatment with aurora kinases inhibitors.
Previous studies have described close interaction between AURKA and Survivin, a nuclear- and cytoplasmic-localised protein that inhibits apoptosis and regulates mitosis [52]. Overexpression of AURKB is associated with poor prognosis in other tumor types [53–55]. In concordance with our OTSCC series, one analysis of 40 oral squamous cell carcinomas showed an association between AURKB and poorly differentiated phenotype, increased multinuclear cells, lymph node metastases and cellular proliferation [56]. Additionally, increased AURKB expression correlated with increased nuclear Survivin expression, which further support our findings. However, in our study we did not find a statistically significant association between AURKB and survival. This leads to the conclusion that the detailed mechanism underlying the effect AURKB overexpression remains unknown.
Conclusion
In conclusion, our findings suggest that Aurora kinase overexpression is a common abnormality in OTSCC and may play a role in its prognosis. We showed areciprocal relationship between AURKA and p53, and between AURKB and Survivin, which might be useful as prognostic factors for OTSCC patients and may have important implications for anticancer targeted therapies.
Acknowledgements
This research was supported by the grant from Mutua Madrilena Grant [2012/0082]. We thank our colleagues from the Radiation Oncology Department who provided insight and expertise that greatly improved the manuscript. We would also like to express gratitude to Professor Kevin Harrington from The Institute of Cancer Research in London for his comments on an early version of the manuscript.
References
- Ng JH, Iyer NG, Tan MH, Edgren G. Changing epidemiology of oral squamous cell carcinoma of the tongue: A global study. Head Neck. 2017;39(2):297–304.
- Patel SC, Carpenter WR, Tyree S, Couch ME, Weissler M, Hackman T, et al. Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18 to 44 years. J Clin Oncol. 2011;29(11):1488–94.
- Rusthoven K, Ballonoff A, Raben D, Chen C. Poor prognosis in patients with stage I and II oral tongue squamous cell carcinoma. Cancer. 2008;112(2):345–51.
- Van Dijk BAC, Brands MT, Geurts SME, Merkx MAW, Roodenburg JLN. Trends in oral cavity cancer incidence, mortality, survival and treatment in the Netherlands. Int J Cancer. 2016;139(3):574–83.
- Zhang H, Dziegielewski PT, Biron VL, Szudek J, Al-Qahatani KH, OConnell DA, et al. Survival outcomes of patients with advanced oral cavity squamous cell carcinoma treated with multimodal therapy: a multi-institutional analysis. J Otolaryngol Head Neck Surg. 2013;42(1):30.
- Mehra R, Serebriiskii IG, Burtness B, Astsaturov I, Golemis EA. Aurora kinases in head and neck cancer. Lancet Oncol. 2013;14(10):e425–35.
- Mahen R, Venkitaraman AR. Pattern formation in centrosome assembly. Curr Opin Cell Biol. 2012;24(1):14–23.
- Nikonova S, Astsaturov I, Serebriiskii IG, Dunbrack RL, Golemis EA. Aurora A kinase (AURKA) in normal and pathological cell division. Cell Mol Life Sci. 2013;70(4):661–87.
- Chen SS, Chang PC, Cheng YW, Tang FM, Lin YS. Suppression of the STK15 oncogenic activity requires a transactivation-independent p53 function. EMBO J. 2002;21(17):4491–9.
- Hauf S, Cole RW, LaTerra S, Zimmer C, Schnapp G, Walter R, et al. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol. 2003;161(2):281–94.
- Carpinelli P, Moll J. Aurora kinase inhibitors: identification and preclinical validation of their biomarkers. Expert Opin Ther Targets. 2008;12(1):69–80.
- Gully CP, Velazquez-Torres G, Shin JH, Fuentes-Mattei E, Wang E, Carlock C, et al. Aurora B kinase phosphorylates and instigates degradation of p53. Proc Natl Acad Sci USA. 2012;109(24):e1513–22.
- Zhang H, Chen X, Jin Y, Liu B, Zhou L. Overexpression of Aurora-A promotes laryngeal cancer progression by enhancing invasive ability and chromosomal instability. Eur Arch Otorhinolaryngol. 2012;269(2):607–14.
- Mazumdar A, Henderson YC, El-Naggar AK, Sen S, Clayman GL. Aurora kinase A inhibition and paclitaxel as targeted combination therapy for head and neck squamous cell carcinoma. Head Neck. 2009;31(5):625–34.
- Tatsuka M, Sato S, Kitajima S, Suto S, Kawai H, Miyauchi M, et al. Overexpression of Aurora-A potentiates HRAS-mediated oncogenic transformation and is implicated in oral carcinogenesis. Oncogene. 2005;24(6):1122–7.
- Reiter R, Gais P, Jütting U, Steuer-Vogt MK, Pickhard A, Bink K, et al. Aurora kinase A messenger RNA overexpression is correlated with tumor progression and shortened survival in head and neck squamous cell carcinoma. Clin Cancer Res. 2006;12(17):5136–41.
- Romain C, Paul P, Kim KW, Lee S, Qiao J, Chung DH. Targeting Aurora kinase-A downregulates cell proliferation and angiogenesis in neuroblastoma. J Pediatr Surg. 2014;49(1):159–65.
- Zhu XP, Liu ZL, Peng AF, Zhou YF, Long XH, Luo QF, et al. Inhibition of Aurora-B suppresses osteosarcoma cell migration and invasion, Exp Ther Med. 2014;7(3):560–4.
- Diaz RJ, Golbourn B, Shekarforoush M, Smith CA, Rutka JT. Aurora kinase B/C inhibition impairs malignant glioma growth in vivo. J Neurooncol. 2012;108(3):349–60.
- Yan M, Wang C, He B, Yang M, Tong M, Long Z, et al. Aurora-A Kinase: A potent oncogene and target for cancer therapy. Med Res Rev. 2016;36(6):1036–1079.
- Bavetsias V, Linardopoulos S. Aurora kinase inhibitors: current status and outlook. Front Oncol. 2015;5:278.
- Tang A, Gao K, Chu L, Zhang R, Yang J, Zheng J. Aurora kinases: novel therapy targets in cancers, Oncotarget. 2017;8(14):23937–54.
- Yang J, Ikezoe T, Nishioka C, Tasaka T, Taniguchi A, Kuwayama Y, et al. AZD1152, a novel and selective aurora B kinase inhibitor, induces growth arrest, apoptosis, and sensitization for tubulin depolymerizing agent or topoisomerase II inhibitor in human acute leukemia cells in vitro and in vivo. Blood. 2007;110(6):2034–40.
- Yang Y, Shen Y, Li S, Jin N, Liu H, Yao X. Molecular dynamics and free energy studies on Aurora kinase A and its mutant bound with MLN8054: insight into molecular mechanism of subtype selectivity. Mol Biosyst. 2012;8(11):3049–60.
- Wang X, Sinn AL, Pollok K, Sandusky G, Zhang S, Chen L, et al. Preclinical activity of a novel multiple tyrosine kinase and aurora kinase inhibitor, ENMD-2076, against multiple myeloma. Br J Haematol. 2010;150(3):313–25.
- Melichar B, Adenis A, Lockhart AC, Bennouna J, Dees EC, Kayaleh O, et al. Safety and activity of alisertib, an investigational aurora kinase A inhibitor, in patients with breast cancer, small-cell lung cancer, non-small-cell lung cancer, head and neck squamous-cell carcinoma, and gastro-oesophageal adenocarcinoma: a five-arm phase 2 study. Lancet Oncol. 2015;16(4):395–405.
- Barr PM, Li H, Spier C, Mahadevan D, LeBlanc M, Ul Haq M, et al. Phase II Intergroup Trial of Alisertib in Relapsed and Refractory Peripheral T-Cell Lymphoma and Transformed Mycosis Fungoides: SWOG 1108. J Clin Oncol. 2015;33(21):2399–404.
- Löwenberg B, Muus P, Ossenkoppele G, Rousselot P, Cahn JY, Ifrah N, et al. Phase 1/2 study to assess the safety, efficacy, and pharmacokinetics of barasertib (AZD1152) in patients with advanced acute myeloid leukemia. Blood. 2011;118(23):6030–6.
- Kantarjian HM, Martinelli G, Jabbour EJ, Quintás-Cardama A, Ando K, Bay JO, et al. Stage I of a phase 2 study assessing the efficacy, safety, and tolerability of barasertib (AZD1152) versus low-dose cytosine arabinoside in elderly patients with acute myeloid leukemia. Cancer. 2013;119(14):2611–9.
- Seymour JF, Kim DW, Rubin E, Haregewoin A, Clark J, Watson P, et al. A phase 2 study of MK-0457 in patients with BCR-ABL T315I mutant chronic myelogenous leukemia and philadelphia chromosome-positive acute lymphoblastic leukemia. Blood Cancer J. 2014;4(8):e238.
- Geuns-Meyer S, Cee VJ, Deak HL, Du B, Hodous BL, Nguyen HN, et al. Discovery of N-(4-(3-(2-aminopyrimidin-4-yl)pyridin-2-yloxy)phenyl)-4-(4-methylthiophen-2-yl)phthalazin-1-amine (AMG 900), a highly selective, orally bioavailable inhibitor of aurora kinases with activity against multidrug-resistant cancer cell lines. J Med Chem. 2015;58(13):5189–207.
- Borthakur G, Dombret H, Schafhausen P, Brummendorf TH, Boissel N, Jabbour E, et al. A phase I study of danusertib (PHA-739358) in adult patients with accelerated or blastic phase chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia resistant or intolerant to imatinib and/or other second generation c-ABL therapy. Haematologica. 2015;100(7):898–904.
- Schöffski P, Besse B, Gauler T, de Jonge MJ, Scambia G, Santoro A, et al. Efficacy and safety of biweekly i.v. administrations of the Aurora kinase inhibitor danusertib hydrochloride in independent cohorts of patients with advanced or metastatic breast, ovarian, colorectal, pancreatic, small-cell and non-small-cell lung cancer: a multi-tumor, multi-institutional phase II study. Ann Oncol. 2015;26(3):598–607.
- Hrabakova R, Kollareddy M, Tyleckova J, Halada P, Hajduch M, Gadher SJ, et al. Cancer cell resistance to aurora kinase inhibitors: identification of novel targets for cancer therapy. J Proteome Res. 2013;12(1):455–69.
- Dees EC, Cohen RB, Von Mehren M, Stinchcombe TE, Liu H, Venkatakrishnan K, et al. Phase I study of aurora A kinase inhibitor MLN8237 in advanced solid tumors: safety, pharmacokinetics, pharmacodynamics, and bioavailability of two oral formulations. Clin Cancer Res. 2012;18(17):4775–84.
- Dees EC, Infante JR, Cohen RB, O’Neil BH, Jones S, Von Mehren M, et al. Phase 1 study of MLN8054, a selective inhibitor of Aurora A kinase in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2011;67(4):945–54.
- Burum-Auensen E, De Angelis PM, Schjølberg AR, Kravik KL, Aure M, Clausen OP. Subcellular localization of the spindle proteins Aurora A, Mad2, and BUBR1 assessed by immunohistochemistry. J Histochem Cytochem. 2007;55(5):477–86.
- Anaka E, Hashimoto Y, Ito T, Okumura T, Kan T, Watanabe G, et al. The clinical significance of Aurora-A/STK15/BTAK expression in human esophageal squamous cell carcinoma. Clin Cancer Res. 2005;11(5):1827–34.
- Klein A, Reichardt W, Jung V, Zang KD, Meese E, Urbschat S., Overexpression and amplification of STK15 in human gliomas. Int J Oncol. 2004;25(6):1789–94.
- Fraizer GC, Diaz MF, Lee IL, Grossman HB, Sen S. Aurora-A/STK15/BTAK enhances chromosomal instability in bladder cancer cells. Int J Oncol. 2004;25(6):1631–9.
- Sen S, Zhou H, Zhang RD, Yoon DS, Vakar-Lopez F, Ito S, et al. Amplification/overexpression of a mitotic kinase gene in human bladder cancer. J Natl Cancer Inst. 2002;94(17):1320–9.
- Jeng YM, Peng SY, Lin CY, Hsu HC. Overexpression and amplification of Aurora-A in hepatocellular carcinoma. Clin Cancer Res. 2004;10(6):2065–71.
- Royce ME, Xia W, Sahin AA, Katayama H, Johnston DA, Hortobagyi G, et al. STK15/Aurora-A expression in primary breast tumors is correlated with nuclear grade but not with prognosis. Cancer. 2004;100(1):12–9.
- Li D, Zhu J, Firozi PF, Abbruzzese JL, Evans DB, Cleary K, et al. Overexpression of oncogenic STK15/BTAK/Aurora A kinase in human pancreatic cancer. Clin Cancer Res. 2003;9(3):991–7.
- Neben K, Korshunov A, Benner A, Wrobel G, Hahn M, Kokocinski F, et al. Microarray-based screening for molecular markers in medulloblastoma revealed STK15 as independent predictor for survival. Cancer Res. 2004;64(9):3103–11.
- Meraldi P, Honda R, Nigg EA. Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53-/- cells. EMBO J. 2002;21(4):483–92.
- Pugacheva EN, Golemis EA. HEF1-aurora A interactions: points of dialog between the cell cycle and cell attachment signaling networks. Cell Cycle. 2006;5(4):384–91.
- Chen SS, Chang PC, Cheng YW, Tang FM, Lin YS. Suppression of the STK15 oncogenic activity requires a transactivation-independent p53 function. EMBO J. 2002;21(17):4491–9.
- Katayama H, Sasai K, Kawai H, Yuan ZM, Bondaruk J, Suzuki F, et al. Phosphorylation by aurora kinase A induces Mdm2-mediated destabilization and inhibition of p53. Nat Genet. 2004;36(1):55–62.
- Liu Q, Kaneko S, Yang L, Feldman RI, Nicosia SV, Chen J, et al. Aurora-A abrogation of p53 DNA binding and transactivation activity by phosphorylation of serine 215. J Biol Chem. 2004;279(50):52175–82.
- Tong T, Zhong Y, Kong J, Dong L, Song Y, Fu M, et al. Overexpression of Aurora-A contributes to malignant development of human esophageal squamous cell carcinoma. Clin Cancer Res. 2004;10(21):7304–10.
- Bolton MA, Lan W, Powers SE, McCleland ML, Kuang J, Stukenberg PT. Aurora B kinase exists in a complex with survivin and INCENP and its kinase activity is stimulated by survivin binding and phosphorylation. Mol Biol Cell. 2002;13(9):3064–77.
- Katayama H, Ota T, Jisaki F, Ueda Y, Tanaka T, Odashima S, et al. Mitotic kinase expression and colorectal cancer progression. J Natl Cancer Inst. 1999;91(13):1160–2.
- Zeng WF, Navaratne K, Prayson RA, Weil RJ. Aurora B expression correlates with aggressive behaviour in glioblastoma multiforme. J Clin Pathol. 2007;60(2):218–21.
- Sorrentino R, Libertini S, Pallante PL, Troncone G, Palombini L, Bavetsias V, et al. Aurora B overexpression associates with the thyroid carcinoma undifferentiated phenotype and is required for thyroid carcinoma cell proliferation. J Clin Endocrinol Metab. 2005;90(2):928–35.
- Qi G, Ogawa I, Kudo Y, Miyauchi M, Siriwardena BS, Shimamoto F, et al. Aurora-B expression and its correlation with cell proliferation and metastasis in oral cancer. Virchows Arch. 2007;450(3):297–302.
Keywords
Tongue neoplasms; Biomarkers; Aurora kinase A; Aurora kinase B; Tumor suppressor protein p53; Cell division
Cite this article
Pablo Nenclares Pena, Ana Ruiz Alonso, Marina Alonso Riano, Ballestin Caravilla C and Perez-Regadera Gomez JF. Prognostic significance of aurora kinase expression and it’s correlation with other apoptotic-like proteins in oral tongue squamous cell carcinoma. Clin Oncol J. 2020;1(1):1–8.
Copyright
© 2020 Pablo Nenclares Pena. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).